
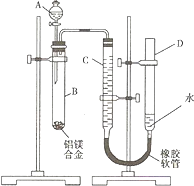
��13�֣�ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������
(1)A���Լ�Ϊ________��
(2)ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����________________ _______��
(3)��ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˣ�ϴ�ӣ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�еμ������Լ����ݼ�������ԡ�����������˳����___ ____(�����)����¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ___ ___��
(4)B�з�����Ӧ�Ļ�ѧ����ʽΪ___________________________________________��
(5)��ʵ������þ�Ͻ������Ϊa g������������Ϊb mL(�ѻ���Ϊ��״��)��B��ʣ����������Ϊc g�����������ԭ������Ϊ__ ______��
(6)ʵ������У���δϴ�ӹ������õIJ����������������������________(�ƫ����ƫС������Ӱ�족)��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2008?���죩ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������
��2008?���죩ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������| 33600(a-c) |
| b |
| 33600(a-c) |
| b |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
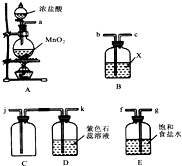 ijѧϰС������ͼװ����ȡ�����������������̽��������
ijѧϰС������ͼװ����ȡ�����������������̽��������
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������A��ΪNaOH��Һ��
ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������A��ΪNaOH��Һ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 �绯ѧ���������״���CH3OH����ˮ����ɵ���Ⱦ����ԭ���ǣ�ͨ���Co2+��������Co3+�����ɵ�Co3+��ˮ�еļ״�������6Co3++CH3OH+H2O=CO2��+6Co2++6H+��ijѧϰС������ͼװ�ý���ʵ�飮����˵��������ǣ�������
�绯ѧ���������״���CH3OH����ˮ����ɵ���Ⱦ����ԭ���ǣ�ͨ���Co2+��������Co3+�����ɵ�Co3+��ˮ�еļ״�������6Co3++CH3OH+H2O=CO2��+6Co2++6H+��ijѧϰС������ͼװ�ý���ʵ�飮����˵��������ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�����ɹŰ�������һ�и���9���¿���ѧ�Ծ����������� ���ͣ�ʵ����
(15��)ijѧϰС������ͼװ�òⶨ��þ�Ͻ������������������������ԭ��������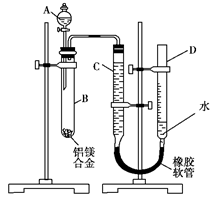
(1)A���Լ�Ϊ ��
(2)ʵ��ǰ���Ƚ���þ�Ͻ���ϡ���н���Ƭ�̣���Ŀ����______________
___________________________________________________________��
(3)��������ԣ���ҩƷ��ˮװ��������У����Ӻ�װ�ú�����еIJ������У��ټ�¼C��Һ��λ�ã��ڽ�B��ʣ�������ˡ�ϴ�ӡ�������أ��۴�B�в���������������ָ������º�¼C��Һ��λ�ã�����A��B�еμ������Լ����ݼ�������ԡ�����������˳������������(�����)����¼C��Һ��λ��ʱ��������ƽ���⣬��Ӧ__________________ ��
(4)B�з�����Ӧ�Ļ�ѧ����ʽΪ__________________________ ��
(5)��ʵ������þ�Ͻ������Ϊa g������������Ϊb mL(�ѻ���Ϊ��״��)��B��ʣ����������Ϊc g�����������ԭ������Ϊ�������� ��
(6)ʵ������У���δϴ�ӹ������õIJ��������������������������������(�ƫ����ƫС����������Ӱ�족)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com