
���ᾧ���Ƭ״���л���У���������������ӽṹ�ɱ�ʾΪ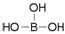 ������������������֯�л��͵ķ������ã��ʿ�����ҽҩ��ʳƷ�����ȷ���
������������������֯�л��͵ķ������ã��ʿ�����ҽҩ��ʳƷ�����ȷ���
��1����������������֪����Ӧ����_____________��
A��ǿ�� B����ǿ�� C������
��2���о��������ڴ��������£�Ԫ�ص�ԭ�����γɷ��ӻ�����ʱ����������Ӳ���дﵽ8�����ȶ��ṹ�����ơ�����������У������ﵽ8�������ȶ��ṹ��ԭ����_______����
��3������ͼ״���Ũ������ڵ������£������ɻӷ�����������������д��������ȫ�����Ļ�ѧ����ʽ��ע����Ӧ������_______________________________________��
��4����֪����0.01mol�ɱ�20mL0.5mol��L-1NaOH��Һǡ����ȫ�кͣ��ݴ��Ʋ⣺������ˮ�������Ե�ԭ���ǣ�д���뷽��ʽ��_________________________________��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
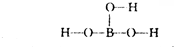
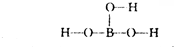
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ᾧ���Ƭ״���л���У���������������ӽṹ�ɱ�ʾΪ������������������֯�л��͵ķ������ã��ʿ�����ҽҩ��ʳƷ�����ȷ���
��1����������������֪����Ӧ����_____________��
A��ǿ�� B����ǿ�� C������
��2���о��������ڴ��������£�Ԫ�ص�ԭ�����γɷ��ӻ�����ʱ����������Ӳ���дﵽ8�����ȶ��ṹ�����ơ�����������У������ﵽ8�������ȶ��ṹ��ԭ����_______����
��3������ͼ״���Ũ������ڵ������£������ɻӷ�����������������д��������ȫ�����Ļ�ѧ����ʽ��ע����Ӧ������_______________________________________��
��4����֪����0.01mol�ɱ�20mL0.5mol��L-1NaOH��Һǡ����ȫ�кͣ��ݴ��Ʋ⣺������ˮ�������Ե�ԭ���ǣ�д���뷽��ʽ��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ���¿α�2010-2011ѧ�����ѧ�ڸ���һ�ָ�ϰ��Ԫ����1�����˽̣� ���ͣ������
���ᾧ���Ƭ״���л���У���������������ӽṹ�ɱ�ʾΪ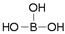 ������������������֯�л��͵ķ������ã��ʿ�����ҽҩ��ʳƷ�����ȷ���
������������������֯�л��͵ķ������ã��ʿ�����ҽҩ��ʳƷ�����ȷ���
��1����������������֪����Ӧ����_____________��
A��ǿ�� B����ǿ�� C������
��2���о��������ڴ��������£� Ԫ�ص�ԭ�����γɷ��ӻ�����ʱ����������Ӳ���дﵽ8�����ȶ��ṹ�����ơ�����������У������ﵽ8�������ȶ��ṹ��ԭ����_______����
Ԫ�ص�ԭ�����γɷ��ӻ�����ʱ����������Ӳ���дﵽ8�����ȶ��ṹ�����ơ�����������У������ﵽ8�������ȶ��ṹ��ԭ����_______����
��3������ͼ״���Ũ������ڵ������£������ɻӷ�����������������д��������ȫ�����Ļ�ѧ����ʽ��ע����Ӧ������_______________________________________��
��4����֪����0.01mol�ɱ�20mL0.5mol��L-1NaOH��Һǡ����ȫ�кͣ��ݴ��Ʋ⣺������ˮ�������Ե�ԭ���ǣ�д���뷽��ʽ��_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ᾧ���Ƭ״���л���У���������������ӽṹ�ɱ�ʾΪ![]() ������������������֯�л��͵ķ������ã��ʿ�����ҽҩ��ʳƷ�����ȷ���
������������������֯�л��͵ķ������ã��ʿ�����ҽҩ��ʳƷ�����ȷ���
��1����������������֪����Ӧ����_____________��
A��ǿ�� B����ǿ�� C������
��2���о��������ڴ��������£�Ԫ�ص�ԭ�����γɷ��ӻ�����ʱ����������Ӳ���дﵽ8�����ȶ��ṹ�����ơ�����������У������ﵽ8�������ȶ��ṹ��ԭ����_______����
��3������ͼ״���Ũ������ڵ������£������ɻӷ�����������������д��������ȫ�����Ļ�ѧ����ʽ��ע����Ӧ������_______________________________________��
��4����֪����0.01mol�ɱ�20mL0.5mol��L-1NaOH��Һǡ����ȫ�кͣ��ݴ��Ʋ⣺������ˮ�������Ե�ԭ���ǣ�д���뷽��ʽ��_________________________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com