
���ڴ�ŵ��������ƿ��ܻᱻ�����е�����������ij��ѧ��ȤС��ͨ��ʵ�����ⶨij��ˮ���������Լ��Ĵ��ȣ����������ʵ�飺
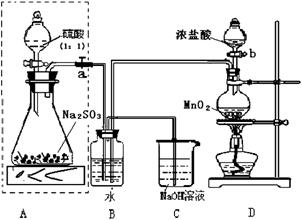
��ش��������⣺
��1���������߿��ڵķ�Һ©�����ɳ���©������Ӧ������������߿���װ�õ������ԣ�
��
��2��Bװ���з�Ӧ�����ӷ���ʽΪ ��
��3������agNa2SO3��Ʒ������ƿ�У���Bװ�÷�Ӧ�����Һ�м���������BaCl2��Һ��ַ�Ӧ�����ˡ�ϴ�ӡ�����ð�ɫ����bg����ԭ��Ʒ��Na2SO3����������Ϊ ��
��4��Cװ���з�����Ӧ�Ļ�ѧ����ʽΪ ��
��5�����������Լ�������ˮ��ϡ���ᡢϡ���ᡢBaCl2��Һ��Ba(NO3)2��Һ�������ѡ������Լ������һ�ֲ�ͬ��ʵ�鷽���ⶨ��������ˮ�������Ʊ������ij̶�
��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����(H2C2O4)��һ����Ҫ�Ļ�����Ʒ�����ᾧ�����ɿ���H2C2O4��xH2O��ʾ��Ϊ�˲ⶨxֵ��������ʵ�飺
�ٳ�ȡ1.260 g�����ᾧ�壬�������Ƴ�100 mLˮ��ҺΪ����Һ��
��ȡ25.00mL�����ƵIJ�����Һ������ƿ�ڣ���������ϡH2SO4����Ũ��Ϊ0.1000 mol/L��KMnO4����Һ���еζ����������ķ�ӦΪ���Իش�
2KMnO4+5H2C2O4+3H2SO4=K2SO4+10CO2��+2MnSO4+8H2O
 (1)ʵ������Ҫ�õ��IJ��������У��ζ��� ��100mL��Ͳ���ձ�����ͷ�ιܡ���ƿ�������������л�ȱ�ٵ�������(�����Ƽ����) ��
(1)ʵ������Ҫ�õ��IJ��������У��ζ��� ��100mL��Ͳ���ձ�����ͷ�ιܡ���ƿ�������������л�ȱ�ٵ�������(�����Ƽ����) ��
(2) �ζ�ʱ����KMnO4��Һװ����ͼ�е�__________(��ס����ҡ�)�ζ����С��ﵽ�ζ��յ�������� ��
(3)�ڵζ�����������ȥ0.1000 mol/L��KMnO4��Һ10.00 mL����ȡ����ƽ��
ֵ�����������ƵIJ�����Һ�����ʵ���Ũ��Ϊ mol/L���ɴ˿ɼ���x��ֵ
�� ��
(4) �����ڽӽ��ζ��յ�ʱ������������ˮ����ƿ�ڱڳ�ϴһ�£��ټ����ζ����յ㣬�������xֵ�� ���ƫ����ƫС������Ӱ�족����ͬ)��
�����ζ��յ�ʱ���Ӷ��������ɴ˲�õ�xֵ��__________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʵ������ͬһԭ�����͵��ǣ�������
A��Ũ�������ˮ����ɫ�Լ�ƿ����
B��Na2S��Na2SO3���峤�ڱ�¶�ڿ����б���
C�����������Ͳ���������Ũ����
D��SO2��Na2SO3��Һ����ʹ��ˮ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵������ȷ���ǣ�NA��ʾ�����ӵ��������� ��
A�������£�2.7 g �������������ᷴӦ���õ��ĵ�����Ϊ0.3NA
B����״���£�44.8LSO2��22.4LO2�ڴ��������·�Ӧ��һ������2NA��SO3
C�����³�ѹ�£�16g�������е���ԭ����ĿΪNA
D. ͨ��״���£�11.2LCO2���еķ�����Ϊ0.5NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����������ʮ������Ԫ���У���һ�ֳ�Ϊ������Ԫ�ء���RԪ�أ����ӳ���������������Ҫ�����á���֪RԪ�ص�ԭ�����ĸ����Ӳ㣬����������ﻯѧʽΪRO3����RԪ�ص�����Ϊ�� ��
A��������������B���顡������������ C������������������D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ṹΪ��-CH=CH-CH��CH-CH=CH-CH=CH-���ĸ߷��ӻ������õ������������䵼�����������ߡ������߷��ӻ�����ĵ�����
A����Ȳ B����ϩ C����ϩ D��1��3-����ϩ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij��ˮ�к��е�NH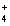 ��һ�������¿ɱ�O2��������Ӧ�������£�
��һ�������¿ɱ�O2��������Ӧ�������£�
�� NH4+(aq)+3/2O2(g)=NO2-(aq)+2H+(aq)+H2O(l) ��H = -273kL/mol
�� NO2-(aq)+1/2O2(g)=NO3-(aq) ��H = -73kL/mol
������������ȷ����
A�� 1mol��NH3��1mol ��NH4+����10��6.02��1023������
B�� �����£�0.1 mol/L HNO2(aq) pH��1����NaNO2��Һ�Լ���
C�� NH4+(aq)+2O2(g)=NO3-(aq)+2H+(aq)+H2O(l) ��H=-346kJ/mol
D�� ����������ת�������У���ˮ����������ǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
C��ʯī��=C�����ʯ������H��+ 1.895kJ��mol ����ͬ�����£�����˵����ȷ���ǣ� ��
A��ʯī�Ƚ��ʯ�ȶ� ����
B�����ʯ��ʯī�ȶ�
C����ͬ���ʵ�����ʯī�Ƚ��ʯ���������� ����
D������Ϊͬλ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
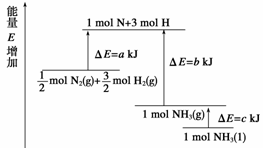
��ѧ��ӦN2��3H2 2NH3�������仯����ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�
2NH3�������仯����ͼ��ʾ���÷�Ӧ���Ȼ�ѧ����ʽ�ǣ�
A��N2(g)��3H2(g)  2NH3(l) ��H��2(a��b��c) kJ��mol��1
2NH3(l) ��H��2(a��b��c) kJ��mol��1
B��N2(g)��3H2(g)  2NH3(g) ��H��2(b��a) kJ��mol��1
2NH3(g) ��H��2(b��a) kJ��mol��1
C.  N2(g)��
N2(g)��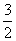 H2(g)
H2(g)  NH3(l) ��H��(b��c��a) kJ��mol��1
NH3(l) ��H��(b��c��a) kJ��mol��1
D.  N2(g)��
N2(g)��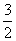 H2(g)
H2(g)  NH3(g) ��H��(a��b) kJ��mol��1
NH3(g) ��H��(a��b) kJ��mol��1
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com