
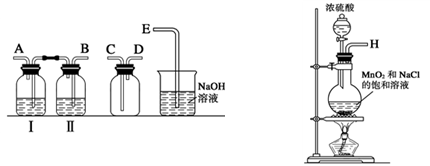
| ���� | ���� |
| ȡ4 gƯ�۾����壬����100 mLˮ | ���ֹ����ܽ⣬��Һ������ɫ |
| ���ˣ���Ư�۾���Һ��pH | pH��ֽ�ȱ�����ԼΪ12��������ɫ |
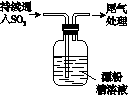 A | ��.Һ���Ϸ�������״�� ��.�Ժ��ֻ��ǣ���Һ��Ϊ����ɫ ��.�Ժ���������ɫ����������ɫ��ȥ |
 �ķ����ǣ��� ����������������������������������������
�ķ����ǣ��� ����������������������������������������

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
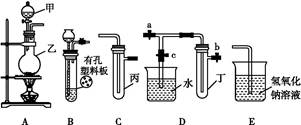
| ʵ����� | ʵ����� | ���� | ���� |
| �� | ����ˮ����Ʒ����Һ | ��Һ��ɫ | ������ˮ��Ӧ�IJ�����Ư���� |
| �� | ��ˮ�м��� NaHCO3��ĩ | ����ɫ���ݲ��� | ������ˮ��Ӧ�IJ�����н�ǿ������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Cl2�ڻ�ѧ��Ӧ�м���������������������ԭ�� |
| B��������ˮ�����ԣ������еμ�������ɫʯ����Һ���������Һ�ʺ�ɫ |
| C��Cl2ͨ�뵽���з�̪��NaOH��Һ�У���ɫ��ȥ����Ϊ������Ư���� |
| D����SO2ͨ����������Һ�����ɴ����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| ѡ�� | X | Y |
| A | SO2 | Cl2 |
| B | NO2 | SO2 |
| C | NH3 | CO2 |
| D | Cl2 | CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
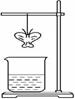
| | A | B | C | D |
| ֽ�����ϵ�����Һ | ��̪ | KI-������Һ | ʯ�� | Ʒ�� |
| С�ձ��е���Һ | Ũ��ˮ | Ũ��ˮ | Ũ���� | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ֻ�Тڢ� | B��ֻ�Тܢ� | C��ֻ�Тڢܢ� | D��ȫ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ף��ң��� | B���ף������� | C�������ף��� | D���ң������� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com