
 ]2-Na+��A��B��C��D����Ԫ�ؿ��γɻ������ң��ҷ���ˮ��������Һ�Լ��ԣ�����������ΪNaHCO3��CH3COONa�ȣ�Ϊǿ�������Σ�ˮ��ʼ��ԣ�����NaHCO3Ϊ���CH3ONaΪ��Է���������С���л��
]2-Na+��A��B��C��D����Ԫ�ؿ��γɻ������ң��ҷ���ˮ��������Һ�Լ��ԣ�����������ΪNaHCO3��CH3COONa�ȣ�Ϊǿ�������Σ�ˮ��ʼ��ԣ�����NaHCO3Ϊ���CH3ONaΪ��Է���������С���л��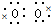 ]2-Na+��NaHCO3��CH3ONa��
]2-Na+��NaHCO3��CH3ONa�� =0.1mol��ת�Ƶĵ��ӵ����ʵ���Ϊ0.2mol������ӵĸ���Ϊ0.2NA��1.204×1023��
=0.1mol��ת�Ƶĵ��ӵ����ʵ���Ϊ0.2mol������ӵĸ���Ϊ0.2NA��1.204×1023��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����¶� Ũ�� |
a���� 400�� |
b���� 425�� |
c���� 450�� |
d���� 475�� |
e���� 500�� |
| c��O2�� | 0.8 | 0.6 | 0.3 | 0.5 | 0.7 |
| c��SO3�� | 0.4 | 0.8 | 1.4 | 1.0 | 0.6 |

| c2(SO3) |
| c2(SO2)��c(O2) |
| c2(SO3) |
| c2(SO2)��c(O2) |


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�



| ��O3 | ��Zn��H2O |




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ�������
Ԫ�����ڱ������ڵ�һ���������ʾ�������й�A��B��C��D��E����Ԫ�ص������У���ȷ���ǣ��������鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com