
�밴�������ʵ�鲽���Ʊ������������壬���齺����Ʊ����������ձ�ȡ��������ˮ���þƾ��Ƽ��������ڣ�Ȼ��������ձ��еμ�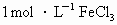 ��Һ�������������Һ������ĺ��ɫ���������������壮
��Һ�������������Һ������ĺ��ɫ���������������壮
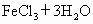
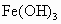 (����)��3HCl
(����)��3HCl
(1)�����������Ƿ��Ƶ��˽��壿
(2)ijͬѧ�ڻ�����У��ߵμ�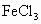 ��Һ���ò��������裬���û���Ƴ����壬��������������е�ԭ��
��Һ���ò��������裬���û���Ƴ����壬��������������е�ԭ��
(3)ȡ�Ƶõ�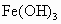 ���������Թ��У����Թ��еμ�һ����ϡ���ᣬ�ߵα����ɿ�����Һ�к��ɫ��dz�����յõ�______ɫ����Һ�������˱仯�Ļ�ѧ����ʽΪ________________________���˷�Ӧ����_________��Ӧ��
���������Թ��У����Թ��еμ�һ����ϡ���ᣬ�ߵα����ɿ�����Һ�к��ɫ��dz�����յõ�______ɫ����Һ�������˱仯�Ļ�ѧ����ʽΪ________________________���˷�Ӧ����_________��Ӧ��
 �ŵ������ϵ�д�
�ŵ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ձ�ȡ��������ˮ���þƾ��Ƽ��ȵ����ڣ�Ȼ�������ձ��еμ�1 mol��L-1��FeCl3��Һ����������е�Һ������ĺ��ɫ���������������塣
FeCl3![]() Fe(OH)3(����)+3HCl
Fe(OH)3(����)+3HCl
(1)�跨��һ���ɼ���ͨ�������Ƶõ�Һ�壬�����Ƿ��ǽ��塣����֮�⣬����Ϊ��������ʲô�����ж���Ľ����Ʊ��Ƿ�ɹ���?
(2)ijͬѧ�ڻ�����У��ߵμ�FeCl3��Һ���ò��������裬���û���Ƴ����壬��������������е�ԭ��????????????
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ձ�ȡ��������ˮ���þƾ��Ƽ��ȵ����ڣ�Ȼ�������ձ��еμ�1 mol��L��1 FeCl3��Һ����������е�Һ������ĺ��ɫ���������������塣?
FeCl3+3H2O![]() Fe(OH)3(����)+3HCl?
Fe(OH)3(����)+3HCl?
��1���跨��һ���ɼ���ͨ�������Ƶõ�Һ�壬�����Ƿ��ǽ��塣����֮�⣬����Ϊ��������ʲô�����ж����Ʊ��Ľ����Ƿ�ɹ���?
��2��ijͬѧ�ڻ�����У��ߵμ�FeCl3��Һ���ò��������裬���û���Ƴ����壬��������������е�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ձ�ȡ��������ˮ���þƾ��Ƽ��ȵ����ڣ�Ȼ�������ձ��еμ�1 mol��L-1FeCl3��Һ����������е�Һ������ĺ��ɫ���������������塣
FeCl3+3H2O![]() Fe(OH)3(����)+3HCl
Fe(OH)3(����)+3HCl
��1���跨��һ���ɼ���ͨ�������Ƶõ�Һ�壬�����Ƿ��ǽ��塣����֮�⣬����Ϊ��������ʲô�����ж���Ľ����Ʊ��Ƿ�ɹ���
��2��ijͬѧ�ڻ�����У��ߵμ�FeCl3��Һ���ò��������裬���û���Ƴ����壬��������������е�ԭ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�밴�������ʵ�鲽���Ʊ������������壬���齺����Ʊ���?
�������ձ�ȡ��������ˮ���þƾ��Ƽ��ȵ����ڣ�Ȼ�������ձ��еμ�1 mol��L-1��FeCl3��Һ����������е�Һ������ĺ��ɫ���������������塣
FeCl3![]() Fe(OH)3(����)+3HCl
Fe(OH)3(����)+3HCl
(1)�跨��һ���ɼ���ͨ�������Ƶõ�Һ�壬�����Ƿ��ǽ��塣����֮�⣬����Ϊ��������ʲô�����ж���Ľ����Ʊ��Ƿ�ɹ���?
(2)ijͬѧ�ڻ�����У��ߵμ�FeCl3��Һ���ò��������裬���û���Ƴ����壬��������������е�ԭ��????????????
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ͬ���� ���ͣ�ʵ����
 Fe( OH)3�����壩+3HCl
Fe( OH)3�����壩+3HCl �鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com