
Ϊ��Ԥ����ȱ�������йز��Ź涨ʳ���еĵ⺬������I�ƣ�Ӧ��20~50 mg/Kg���Ʊ�KIO3�ķ������£�
����1��6I2��11KClO3��3H2O��6KH(IO3)2��5KCl��3Cl2����KH(IO3)2��KOH��2KIO3��H2O
����2�����������£�
����3��
���뷽��3��ȷ���1�IJ����� ������2�IJ��������� ��
�Ʒ���2ѡ�õĵ缫�Ƕ��Ե缫������������Ӧʽ�� ��
�Ƿ���3��Ӧ�¶ȿ�����70�����ң������ø����¶ȵ���Ҫԭ���� ��
���Ʊ�����KIO3��ʵ�鲽���У�����轫���þ������ʹ�� ϴ��2~3�Σ������ò�Ʒ��
����֪��KIO3��5KI��3H2SO4=3K2SO4��3I2��3H2O�� I2��2S2O32��=2I����S4O62����Ϊ�ⶨ�ӵ�ʳ���е�ĺ�������Ƶķ������¡�������ʵ�鲽�貢����ⶨ�����
I2��2S2O32��=2I����S4O62����Ϊ�ⶨ�ӵ�ʳ���е�ĺ�������Ƶķ������¡�������ʵ�鲽�貢����ⶨ�����
a��ȷ��ȡw gʳ������ƿ�У��ټ���������ˮʹ����ȫ�ܽ⡣
b�� ��
c������ƿ�еμ�2.0��10��3 mol/L Na2S2O3����Һ���յ㡣
d���ظ�����ʵ�����Ρ�
����ʵ������ݼ�¼���±���������ӵ�ʳ����Ʒ�еĵ�Ԫ�غ����� mg/kg���Ժ�w�Ĵ���ʽ��ʾ����
| �ζ����� | ʢ��Na2S2O3��Һ�Ķ��� | |
| �ζ�ǰ�̶ȣ�/mL�� | �ζ���̶ȣ�/mL�� | |
| 1 | 1.02 | 11.03 |
| 2 | 2.00 | 11.99 |
| 3 | 0.20 | 10.20 |
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| H2O2��H+ |
| 70�� |
| K2CO3 |
| �ζ����� | ʢ��Na2S2O4��Һ�Ķ��� | |
| �ζ�ǰ�̶ȣ�/mL�� | �ζ���̶ȣ�/mL�� | |
| 1 | 1.02 | 11.03 |
| 2 | 2.00 | 11.99 |
| 3 | 0.20 | 10.20 |
| 1270 |
| 3w |
| 1270 |
| 3w |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�022
�ִ������У�����Խ��Խע����Ԫ�ص����룮��Ԫ�ض����彡������������Ҫ�����ã�Ϊ��Ԥ����ȱ�������ҹ��������涨ÿǧ��ʳ����Ӧ����40��50mg����أ�
�����ش��������⣺
������1���ӵ�ʳ�εİ�װ����ͨ����������ʳ�ý��飺��ʱ������������������������Ļ�ѧԭ��________��
������2��![]() ���õ�ⷽ���Ƶã�ԭ���ǣ���ʯīΪ�������Բ����Ϊ��������һ���¶Ⱥ͵���ǿ���µ��KI��Һ���ܷ�Ӧ����ʽΪ��_____________________________________
���õ�ⷽ���Ƶã�ԭ���ǣ���ʯīΪ�������Բ����Ϊ��������һ���¶Ⱥ͵���ǿ���µ��KI��Һ���ܷ�Ӧ����ʽΪ��_____________________________________
����![]() ���������ĵ缫��Ӧʽ�ֱ�Ϊ������________������________��
���������ĵ缫��Ӧʽ�ֱ�Ϊ������________������________��
������3��Ϊ����ijʳ�����Ƿ��е���أ�ijͬѧȡʳ����Ʒ428g��ȫ�ܽ���ˮ�У�Ȼ����������ữ�ĵ��۵⻯����Һ�������Һ����ɫ����Ӧ�����ӷ���ʽΪ________��Ϊ��һ��ȷ������Ʒ�Ƿ�Ϊ�ϸ��Ʒ����������0.030 mol/L�������������Һ�ζ�������ȥ18.00mLʱ��ɫ�պ���ȥ����Ӧ����ʽΪ��![]() �����ɴ�ͨ��������жϸüӵ�ʳ��Ϊ________�������ϸ����������ϸ�������Ʒ��
�����ɴ�ͨ��������жϸüӵ�ʳ��Ϊ________�������ϸ����������ϸ�������Ʒ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
�����ش��������⣺
������1���ӵ�ʳ�εİ�װ����ͨ����������ʳ�ý��飺��ʱ������������������������Ļ�ѧԭ��________��
������2��![]() ���õ�ⷽ���Ƶã�ԭ���ǣ���ʯīΪ�������Բ����Ϊ��������һ���¶Ⱥ͵���ǿ���µ��KI��Һ���ܷ�Ӧ����ʽΪ��_____________________________________
���õ�ⷽ���Ƶã�ԭ���ǣ���ʯīΪ�������Բ����Ϊ��������һ���¶Ⱥ͵���ǿ���µ��KI��Һ���ܷ�Ӧ����ʽΪ��_____________________________________
����![]() ���������ĵ缫��Ӧʽ�ֱ�Ϊ������________������________��
���������ĵ缫��Ӧʽ�ֱ�Ϊ������________������________��
������3��Ϊ����ijʳ�����Ƿ��е���أ�ijͬѧȡʳ����Ʒ428g��ȫ�ܽ���ˮ�У�Ȼ����������ữ�ĵ��۵⻯����Һ�������Һ����ɫ����Ӧ�����ӷ���ʽΪ________��Ϊ��һ��ȷ������Ʒ�Ƿ�Ϊ�ϸ��Ʒ����������0.030 mol/L�������������Һ�ζ�������ȥ18.00mLʱ��ɫ�պ���ȥ����Ӧ����ʽΪ��![]() �����ɴ�ͨ��������жϸüӵ�ʳ��Ϊ________�������ϸ����������ϸ�������Ʒ��
�����ɴ�ͨ��������жϸüӵ�ʳ��Ϊ________�������ϸ����������ϸ�������Ʒ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2004ȫ����ʡ�и߿�ģ�������ࡤ��ѧ ���ͣ�043
�ִ������У�����Խ��Խע����Ԫ�ص����룮��Ԫ�ض����彡������������Ҫ�����ã�Ϊ��Ԥ����ȱ�������ҹ��������涨ÿǧ��ʳ����Ӧ����40��50mg����أ�
�ش��������⣺
(1)�ӵ�ʳ�εİ�װ����ͨ����������ʳ�ý��飺��ʱ������������������������Ļ�ѧԭ��________��
(2)KIO3���õ�ⷽ���Ƶã�ԭ���ǣ���ʯīΪ�������Բ����Ϊ��������һ���¶Ⱥ͵���ǿ���µ��KI��Һ���ܷ�Ӧ����ʽΪ��
KI��3H2O KIO3��3H2�����������ĵ缫��Ӧʽ�ֱ�Ϊ������________������________��
KIO3��3H2�����������ĵ缫��Ӧʽ�ֱ�Ϊ������________������________��
(3)Ϊ����ijʳ�����Ƿ��е���أ�ijͬѧȡʳ����Ʒ428g��ȫ�ܽ���ˮ�У�Ȼ����������ữ�ĵ��۵⻯����Һ�������Һ����ɫ����Ӧ�����ӷ���ʽΪ________��Ϊ��һ��ȷ֤����Ʒ�Ƿ�Ϊ�ϸ��Ʒ����������0.030mol/L�������������Һ�ζ�������ȥ18.00mLʱ��ɫ�պ���ȥ(��Ӧ����ʽΪ��I2��2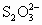 ��2I����
��2I���� )���ɴ�ͨ��������жϸüӵ�ʳ��Ϊ________(��ϸ��ϸ�)��Ʒ��
)���ɴ�ͨ��������жϸüӵ�ʳ��Ϊ________(��ϸ��ϸ�)��Ʒ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(2)�����У�����Խ��Խע����Ԫ�ص����룬��Ԫ�ض����彡���뷢������������Ҫ�����á�Ϊ��Ԥ����ȱ�������йز��Ź涨ÿǧ��ʳ����Ӧ����40��50 mg �ĵ���ء���֪����ؿ��õ�ⷽ���Ƶã���ʯīΪ�����������Ϊ��������һ���ĵ���ǿ�Ⱥ��¶��µ��KI��Һ���ܷ�Ӧ��ѧ����ʽΪKI+3H2O![]() KIO3+3H2����������Ӧʽ��______________��
KIO3+3H2����������Ӧʽ��______________��
(3)����̽��ڻ��Ǵ����м�������A��������ʾ��A��������ԭ�ӷ��ӣ�����Է�������Ϊ60���ڵ�����A�ֽ⡣��ĩ״��KSCN��Ũ������һ�������¿ɵõ�����A�������������Σ�����������ʵ���֮����1��1��1��������A�Ľṹʽ��___________��
(4)���������ˮ���о��IJ������룬ˮ��Ӧ��ҲԽ��Խ�㷺���о���Ա������֣���һ����ʵ�������£���ˮʩ��һ�����糡����
A.����ɱ� B.��ֹ�����ۻ�
C.���������������� D.�����������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com