
��12�֣����̿����Ҫ�ɷ���̼���̣����� Fe2O3��FeO ��CaO��MgO �ȳɷ֡�ij�������÷����ᣨ��������Լ20%�������̿��Ʊ�MnCl2��4H2O��106��ʱʧȥһ���ӽᾧˮ��198��ʱʧȥȫ���ᾧˮ������ˮ������ֹ����������£�

��1������������þ����̼���̷�Ӧ�Ļ�ѧ����ʽΪ��_______________________________��
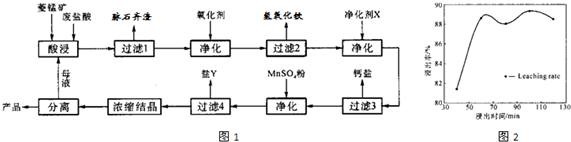
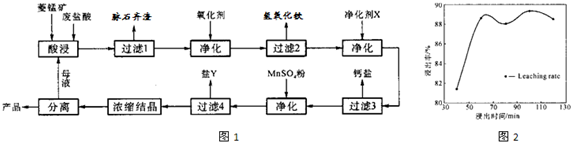
��2�����ʱ������ʱ����̽����ʵ�Ӱ������ͼ����ҵ���õ��ǽ�ȡ60min�������ԭ���ǣ�____________________________________________________��
��3��ͼ�С�������X��������________________________��
��4������4��õ���Y������Ҫ�ɷ���__________________________��
��5��Ũ���ᾧʱ������һ���־�Ĥ����ֹͣ���ȣ���ԭ���ǣ�_______________________��
��1��MnCO3+2HCl=MnCl2+CO2��+H2O��3�֣�
��2��60min�����ӳ�����ʱ�����������ɱ��������������������ԡ���3�֣�[��Դ:ѧ&��&��]
��3��MnCO3��2�֣�
��4������þ��MgSO4��7H2O����2�֣�
��5��ˮ�ݺ���ʱ�����յõ��Ŀ�����ʧȥ���ֽᾧˮ���Ȼ��̡���2�֣�
����������1��̼���̺����ᷴӦ���������Ȼ��̡�ˮ��CO2����ѧ����ʽΪ
MnCO3+2HCl=MnCl2+CO2��+H2O��
��2������ͼ���֪��60min�����ӳ�����ʱ�����������������ԣ����һ������������ɱ���
��3����Ϊ���������µ����ʣ����ԡ�������X����MnCO3��
��4������ת��ͼ��֪����ʱ������Һ�е������ӳ����������⣬��Ҫ����þ���ӣ�����Y������þ��
��5��MnCl2��4H2O�ڼ���ʱ����ʧȥ�ᾧˮ�����²�Ʒ������


 ���ݼ���ϵ�д�
���ݼ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��12�֣����̿����Ҫ�ɷ���̼���̣����� Fe2O3��FeO ��CaO��MgO �ȳɷ֡�ij�������÷����ᣨ��������Լ20%�������̿��Ʊ�MnCl2��4H2O��106��ʱʧȥһ���ӽᾧˮ��198��ʱʧȥȫ���ᾧˮ������ˮ������ֹ����������£�
��1������������þ����̼���̷�Ӧ�Ļ�ѧ����ʽΪ��_______________________________��
��2�����ʱ������ʱ����̽����ʵ�Ӱ������ͼ����ҵ���õ��ǽ�ȡ60min�������ԭ���ǣ�____________________________________________________��
��3��ͼ�С�������X��������________________________��
��4������4��õ���Y������Ҫ�ɷ���__________________________��
��5��Ũ���ᾧʱ������һ���־�Ĥ����ֹͣ���ȣ���ԭ���ǣ�_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�콭��ʡ�����߿�ѹ�ữѧ�Ծ� ���������� ���ͣ������
��12�֣����̿����Ҫ�ɷ���̼���̣����� Fe2O3��FeO��CaO��MgO �ȳɷ֡�ij�������÷����ᣨ��������Լ20%�������̿��Ʊ�MnCl2��4H2O��106��ʱʧȥһ���ӽᾧˮ��198��ʱʧȥȫ���ᾧˮ������ˮ������ֹ����������£�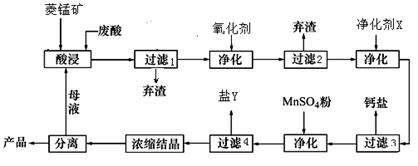
��1������������þ����̼���̷�Ӧ�Ļ�ѧ����ʽΪ��_______________________________��
��2�����ʱ������ʱ����̽����ʵ�Ӱ������ͼ����ҵ���õ��ǽ�ȡ60min�������ԭ���ǣ�____________________________________________________��
��3��ͼ�С�������X��������________________________��
��4������4��õ���Y������Ҫ�ɷ���__________________________��
��5��Ũ���ᾧʱ������һ���־�Ĥ����ֹͣ���ȣ���ԭ���ǣ�_______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com