
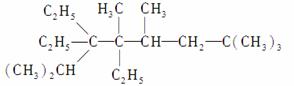
(1)д�����л���������ƻ�ṹ��ʽ��
��
________________________________________________________________________��
��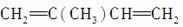 ________________________________________________________________________��
________________________________________________________________________��
��CH2===CHCOOH________________________________________________________________________��
��2,5���� 2,4����ϩ�Ľṹ��ʽ��
________________________________________________________________________
________________________________________________________________________��
(2)������ֳƻƼ���ҹ��ض�����ҩ������������е�һ���������ҹ���ѧ���о�������ṹ���£�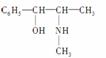
��������к��������ŵ�������________������________��(������ӡ�)��
�����и����ʣ�
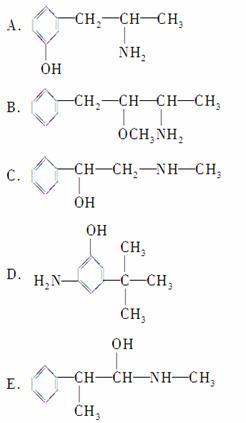
������ػ�Ϊͬ���칹�����________(����ĸ����ͬ)����Ϊͬϵ�����________��
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д� ��˼άС�ھ�100����ҵ��ϵ�д�
��˼άС�ھ�100����ҵ��ϵ�д� ��ʦָ��һ��ͨϵ�д�
��ʦָ��һ��ͨϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������ײʱ����ȫ�����з�����Ӧ:10NaN3+2KNO3= K2O+5Na2O+16N2��������������Ȼ�ԭ�����1. 75 mol,�������ж���ȷ����
A.����40.0 L N2(��״��) B.��0. 250 mol KNO3������
C.ת�Ƶ��ӵ����ʵ���Ϊ1. 75mol D.��������Nԭ�ӵ����ʵ���Ϊ3.75mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���Ľṹ��ʽΪ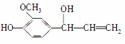 ���������ŷ��࣬��������(����)
���������ŷ��࣬��������(����)
A��ϩ�� B������
C������ D������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ�ȴ�����2�֣����ȴ�����4�ֵ�����(����)
A������ B��2������
C����ϩ D����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�л���3.24 gװ��Ԫ�ط���װ�ã�ͨ������������ʹ֮��ȫȼ�գ������ɵ���������ͨ����ˮCaCl2(A)�ܺͼ�ʯ��(B)�ܣ����A������2.16 g��B������9.24 g����֪���л������Է�������С��200������л����ʵ��ʽ�ͻ�ѧʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���������м��������ᷴӦ��������NaOH��Һ��Ӧ����(����)
��NaHCO3����(NH4)2S����Al(OH)3����NH4Cl
��H2N��CH2��COOH����CH3COOH
A���٢ڢ� B���٢ڢܢ�
C���ݢ� D���٢ڢۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪ȩ��һ�������·������·�Ӧ��
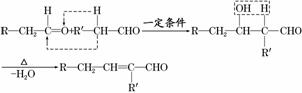
�ɿ���ȩ�ϳ��ö���ȩ�Ĵ�ͳ�ϳ�·����ͼ��ʾ��
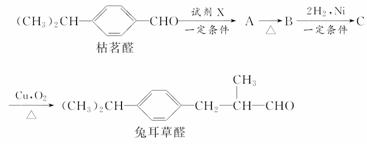
(1)д���Լ�X�Ľṹ��ʽ��
________________________________________________________________________��
(2)д���л���B�Ľṹ��ʽ��
________________________________________________________________________��
(3)д���л���C �D���ö���ȩ�Ļ�ѧ����ʽ��
________________________________________________________________________��
(4)���������������о����ö���ȩ�ĺϳ�·�ߣ���·��ԭ�������������Ͽɴ�100%��
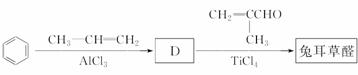
��д��D�Ľṹ��ʽ��____________��
(5)�����廯����Y�����ȩ��Ϊͬ���칹�壬Y��������������
a�����ܷ���������Ӧ���ɷ�����ȥ��Ӧ��
b���˴Ź���������ʾ��Y��ȥ��Ӧ����Ļ���ֻ����һ�ֻ�ѧ��������ԭ�ӡ�д��Y���ܵĽṹ��ʽ��
__________________��__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�о�С������ӷ���ʽxR2+ + yH+ + O2 = mR3+ + nH2O�ķ����о�,����˵���д������
A�����ݵ���غ�,�ó�x��y�ĺ�һ������m
B������ԭ���غ�,�ó�x��m����ֵһ�����
C�����ݵ��ӵ�ʧ�غ�,�ó�x=4�Ľ���
D������������ԭ��Ӧ��ϵ�ó���R2+�ǻ�ԭ��, O2��������, R3+����������, H2O�ǻ�ԭ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com