
(12��)�����仯����������������й㷺Ӧ�á���ش��������⣺
��1��������FeS2�������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ
3FeS2��8O2 6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��ת�� mol���ӡ�
6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��ת�� mol���ӡ�
��2���Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ
��
��3�����������ƣ�������Ҳ��������ˮ������ԭ��Ϊ �������ӷ��̱�ʾ��
��4���ٸ����ĵ绯��ʴ��ʾ��ͼ���£�����ͼ�����ļ��ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ��������ͼ���߿��������ģ����ü�ͷ���������������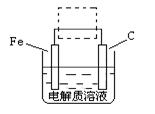
��д����ǰ�ĸ���������ʴʯī�缫�ĵ缫��Ӧʽ ��
��5��������һ�ֺ�ɫ���ϣ���ɷ���Fe2O.3��һ��������������160ml5 mol��L��1�����У��ڼ���һ��������ǡ���ܽ⣬�ռ���2.24L����״����������⣬��ҹ����Fe3+,��μӷ�Ӧ�����۵�����Ϊ ��
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)�����仯����������������й㷺Ӧ�á���ش��������⣺
��1��������FeS2�������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ
3FeS2��8O26SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��ת�� mol���ӡ�
��2���Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ
��
��3�����������ƣ�������Ҳ��������ˮ������ԭ��Ϊ �������ӷ��̱�ʾ��
��4���ٸ����ĵ绯��ʴ��ʾ��ͼ���£�����ͼ�����ļ��ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ��������ͼ���߿��������ģ����ü�ͷ���������������
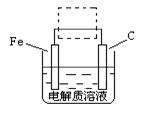
��д����ǰ�ĸ���������ʴʯī�缫�ĵ缫��Ӧʽ ��
��5��������һ�ֺ�ɫ���ϣ���ɷ���Fe2O.3��һ��������������160ml5 mol��L��1�����У��ڼ���һ��������ǡ���ܽ⣬�ռ���2.24L����״����������⣬��ҹ����Fe3+,��μӷ�Ӧ�����۵�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ������ʡ��ʦ���и�һ��ѧ����ĩ���⻯ѧ�������Ծ� ���ͣ������
(15��)�����仯�����ڹ��á��ճ�������ռ�зdz� ��Ҫ�ĵ�λ���˽������仯��������ʺ���;�dz���Ҫ����ش��������⣺
��Ҫ�ĵ�λ���˽������仯��������ʺ���;�dz���Ҫ����ش��������⣺
(1)������Ȼ������ ̬���ڣ���������ʺ�ɫ���� ���ʺ��ɫ���� (��д��ѧʽ)��
(2)���ڴ�������ȼ�յĻ�ѧ����ʽΪ ��
���������з�Ӧ�IJ����� ��
(3)Ҫ��֤һ����Һ���Ƿ���Fe3+��Fe2+����ȷ��ʵ�鷽���� ��
A.����Թ��� ������Һ������KSCN��Һ������Ѫ��ɫ��֤��һ������Fe3+��
������Һ������KSCN��Һ������Ѫ��ɫ��֤��һ������Fe3+��
B.�����Թ��м�����Һ��������ˮ������ˮ��ɫ��֤��һ������Fe2+��
C.����Թ��м�����Һ��������ˮ���ٵ���KSCN��Һ������Ѫ��ɫ��֤��ԭ��Һ��һ������Fe3+
(4)���Ͻ����������ݣ�
| �� | |||
| ̼�ظ�(Fe��C��Mn��Si) | �Ͻ�� | ||
| ��̼�� | ��̼�� | ��̼�� | ̼�ظ�+Cr��Mn��W��Ni��Co�� |
| ��̼��<0.3% | ��̼��0.3%--0.6% | ��̼��>0.6% | |
| ���Ժã�ǿ�ȵ� | ���Ժá�ǿ�Ⱥ� | Ӳ���� | ������������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�콭��ʡ������ѧ�����ڶ��ζο������ۺϻ�ѧ�Ծ����������� ���ͣ������
(16��)�����仯����������������й㷺Ӧ�á���ش��������⣺
��1��������FeS2�������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ
3FeS2��8O2 ==6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��
==6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��
ת�� mol���ӡ�
��2���Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ ��
��3�����������ƣ�������Ҳ��������ˮ������ԭ��Ϊ �������ӷ��̱�ʾ��
��4���ٸ����ĵ绯��ʴ��ʾ��ͼ���ң�����ͼ�����ļ��ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ��������ͼ���߿��������ģ����ü�ͷ���������������
��д����ǰ�ĸ���������ʴʯī�缫�ĵ缫��Ӧʽ ��
��5��������һ�ֺ�ɫ���ϣ���ɷ���Fe2O.3��һ��������������160ml5 mol��L��1�����У��ڼ���һ��������ǡ���ܽ⣬�ռ���2.24L����״����������⣬��ҹ����Fe3+����μӷ�Ӧ�����۵�����Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɳ��һ�п�ǰԤ�����ѧ���� ���ͣ������
(12��)�����仯����������������й㷺Ӧ�á���ش��������⣺
��1��������FeS2�������������ұ����������Ҫԭ�ϡ�����һ����ӦΪ
3FeS2��8O2 6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��ת��
mol���ӡ�
6SO2��Fe3O4����������Ϊ ������3 mol FeS2�μӷ�Ӧ��ת��
mol���ӡ�
��2���Ȼ�����Һ������ӡˢ��·ͭ�帯ʴ������Ӧ�����ӷ���ʽΪ
��
��3�����������ƣ�������Ҳ��������ˮ������ԭ��Ϊ �������ӷ��̱�ʾ��
��4���ٸ����ĵ绯��ʴ��ʾ��ͼ���£�����ͼ������ ���ɳ�Ϊ�����绯ѧ�����ļ�ʾ��ͼ��������ͼ���߿��������ģ����ü�ͷ���������������

��д����ǰ�ĸ���������ʴʯī�缫�ĵ缫��Ӧʽ ��
��5��������һ�ֺ�ɫ���ϣ���ɷ���Fe2O.3��һ��������������160ml5 mol��L��1�����У��ڼ���һ��������ǡ���ܽ⣬�ռ���2.24L����״����������⣬��ҹ����Fe3+,��μӷ�Ӧ�����۵�����Ϊ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com