



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
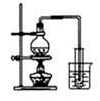 |  | 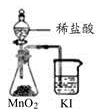 | 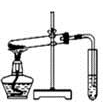 |
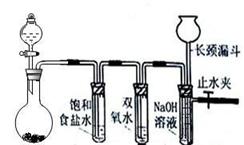
| A����ȡ��������ˮ | B����ȡ����NO2 | C���Ƚ�MnO2��Cl2��I2�������� | D��̽��NH4HCO3�����ȶ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
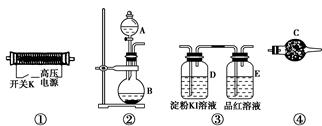
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
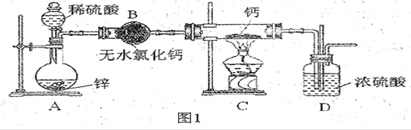
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��NO | B��H2 | C��NH3 | D��CO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
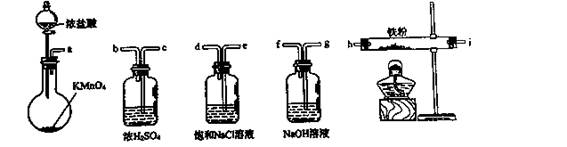
| A��a-b-c-d-e-e-f-g-h | B��a-e-d-c-b-h-i-g |
| C��a-d-e-c-b-h-i-g | D��a-c-b-d-e-h-i-f |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
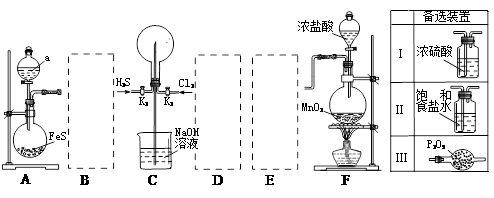
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
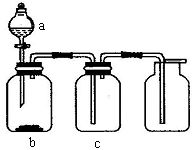
| ѡ�� | ���� | a | b | c |
| A | CO2 | ϡ���� | ʯ��ʯ | Ũ���� |
| B | SO2 | Ũ���� | ��Ƭ | Ũ���� |
| C | NH3 | Ũ��ˮ | ��ʯ�� | ��ʯ�� |
| D | Cl2 | Ũ���� | MnO2��ĩ | Ũ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ǧ���Ʊ�HF | B����Ũ�����Ʊ�HI |
| C����ĥɰ�ӿڵ�װ���Ʊ�HNO3 | D���ñ���ʳ��ˮ����ˮ�Ʊ���Ȳ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com