
| A�� | 0.1mol•L-1NaHC2O4��Һ��0.1mol•L-1KOH��Һ�������ϣ�������Һ�У�c��Na+����c��K+����c��C2O42-����c��OH-����c��HC2O4-�� | |
| B�� | 20mL 0.1 mol•L-1 NH4Cl��Һ��10mL 0.1mol•L-1NaOH��Һ��Ϻ���Һ�ʼ��ԣ�������Һ�У�c��Cl-����c��NH4+����c��Na+����c��OH-����c��H+�� | |
| C�� | �����£�pH=2��HF��Һ��pH=12������������Һ�������ϣ�������Һ�У�c��Na+��+c��OH-����c��H+��+c��F-�� | |
| D�� | 0.1mol•L-1 NH3•H2O��Һ��0.1mol•L-1HNO3�������ϣ�������Һ�У�c��H+����c��OH-��+c��NH3•H2O�� |
���� A������ǡ�÷�Ӧ���ɵ�Ũ�ȵIJ����ơ�����أ���c��Na+��=c��K+������������Ӳ���ˮ�⣬��Һ��ʾ���ԣ�c��Na+��=c��K+����c��C2O42-����
B����Ӧ����Һ������Ϊ��Ũ�ȵ�һˮ�ϰ����Ȼ�狀� �Ȼ��ƣ���Һ�ʼ��ԣ���c��OH-����c��H+����˵��һˮ�ϰ��ĵ���̶ȴ���笠����ӵ�ˮ��̶ȣ�����Һ��笠�����Ũ������c��NH4+����c��Na+����
C�����ݻ��Һ�еĵ���غ��жϣ�
D.0.1mol•L-1 NH3•H2O��Һ��0.1mol•L-1HNO3�������ϣ�����ǡ�÷�Ӧ��������泥������������Һ�е������غ��жϣ�
��� �⣺A.0.1mol•L-1NaHC2O4��Һ��0.1mol•L-1KOH��Һ�������ϣ�����ǡ�÷�Ӧ���ɵ�Ũ�ȵIJ����ơ�����أ�c��Na+��=c��K+����������Һ���������������Բ�������ӵ�ˮ���ˮ�ĵ��룬��c��OH-����c��HC2O4-������Һ����ȷ������Ũ�ȴ�СΪ��c��Na+��=c��K+����c��C2O42-����c��OH-����c��HC2O4-������A����
B.20mL 0.1 mol•L-1 NH4Cl��Һ��10mL 0.1mol•L-1NaOH��Һ��Ϻ���Һ�ʼ��ԣ����ߵĻ����Һ�����ʵ�Ũ�ȵ�NH4Cl��NaCl��NH3•H2O����Һ�ʼ���˵��һˮ�ϰ��Ե���Ϊ������笠�����Ũ������������Һ������Ũ�ȴ�СΪ��c��Cl-����c��NH4+����c��Na+����c��OH-����c��H+������B��ȷ��
C�������£�pH=2��HF��Һ��pH=12������������Һ�������ϣ�������Һ��һ���������غ㣺c��Na+��+c��OH-��=c��H+��+c��F-������C����
D������ǡ����ȫ��Ӧ����NH4NO3�����������غ�ɵã�c��H+��=c��OH-��+c��NH3•H2O������D����
��ѡB��
���� ���⿼��������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ��Ӧ���������Ϊ���ؼ���ע�����յ���غ㡢�����غ㼰�����غ����ж�����Ũ�ȴ�С�е�Ӧ�÷�����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢۢܢݢ� | B�� | �ڢܢݢ� | C�� | �٢ڢۢ� | D�� | �ۢܢݢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ������M�ķ���ʽΪC14H15NO7 | |
| B�� | ������M��ʹ����KMnO4��Һ��ɫ | |
| C�� | ������M�ܷ����ӳɷ�Ӧ�����ܷ�����ȥ��Ӧ | |
| D�� | 1mol��NaOH��Һ�з�Ӧ���������4molNaOH |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Cl2��Al��H2 | B�� | F2��K��HCl | C�� | NO2��Na��Br2 | D�� | HNO3��SO2��H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �������ƺ�����������������θ����� | |
| B�� | ���Ѿ��г�����һ������S02������������ɱ�� | |
| C�� | �����к����������ɲ�������ȱ������Ԫ�� | |
| D�� | ʯ�ͷ����Ŀ����Ϊ�˻����ϩ����ϩ�Ͷ���ϩ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
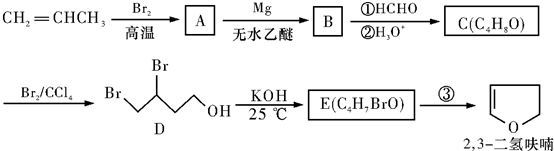
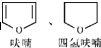
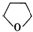 +H2O��
+H2O���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=13��NaOH��Һ�к��е�OH-����Ϊ0.1 NA | |
| B�� | Fe������Cl2��ȼ������0.5 mol ���ת�Ƶĵ�����Ϊ1 NA | |
| C�� | 18g D2O�к��е�������Ϊ9NA | |
| D�� | ��״���£���4 mol HCl��Ũ����������MnO2���ȷ�Ӧ������22.4 L���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �� | B�� | ������� | C�� | ���� | D�� | ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com