
����98��(�ܶ�Ϊ1.84 g/cm3)��Ũ����ϡ�ͳ�3 mol/L��ϡ����100 mL���ش��������⣺
(1)��ҪȡŨ����________mL
(2)���ڴ��������ʹ�õ������ݱ���ȷ����ƫС����________(��д���)��
A������Ͳ��ȡһ����Һ��ʱ������Һ�����
B��ʹ������ƿ������Һʱ������Һ�涨�ݺ�������Һ��Ũ��
C��û��������ˮϴ�ձ�2��3�Σ�����ϴҺ��������ƿ��
D������ƿ��������ˮϴ����û�к��
E������ʱ���μ�����ˮ����ʹҺ���Ը��ڿ̶��ߣ�����������ˮʹҺ�氼����̶�������
F������õ���Һ�����������ˮϴ�����Լ�ƿ�б���
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| b |
| a(1-m) |
| 100b |
| a(1-m%) |
| b |
| a(1-m) |
| 100b |
| a(1-m%) |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ��������ͨ��ѧ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
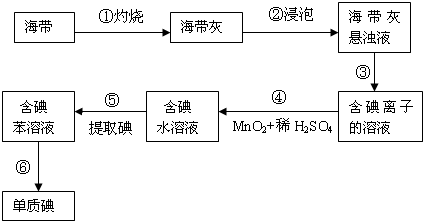
��11�֣������к��зḻ�ĵ⣬Ϊ�˴Ӻ�������ȡ�⣬ij�о���ѧϰС����Ʋ�����������ʵ�飺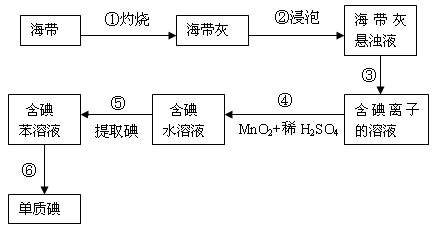
��1������������õ�1mol/L��ϡ����100mL������98%���ܶ�Ϊ1.84g/cm3��Ũ���������ƣ���Ӧ����Ͳ��ȡŨ���������� ��
��2������۵�ʵ����������� �������Ŀ���ǴӺ��ⱽ��Һ�з�������ʵ�ͻ��ձ����ò����ʵ����������� ��
��3���������ѡ���ñ������þƾ�����ȡ��������� ��
��4���������ϣ���С��ͬѧ֪�������еĵ�Ԫ����Ҫ���л��⻯�����ʽ���ڣ�����С��ʵ��ʱ�Ƶú���������Ϊag����ȡ��Ĺ����е���ʧ��m%�����õ����ʵ������Ϊbg����С��ͬѧ��õĺ����е�ĺ���Ϊ���ú�a��b��m��ʽ�ӱ�ʾ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�꼪��ʡ��������ͨ��ѧ��һ���ϣ����л�ѧ�Ծ��������棩 ���ͣ������

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com