
| ���� | �״� | ���װ� | ���������� | ���� | ̼������� |
| �ṹʽ | CH3OH | ��CH3��2NH | ��CH3��2NCHO | CH3OCH | ��CH3O��2CO |
| �е㣨�棩 | 64.7 | 7.4 | 153�� | -24.9 | 90�� |
| ������� | �¶�/�� | ��ʼ���ʵ���/mol | ƽ�����ʵ���/mol | |
| CH3OH | CH3OCH3 | H2O | ||
| �� | 387 | 0.20 | x | |
| �� | 387 | 0.40 | y | |
| �� | 207 | 0.20 | 0.090 | 0.090 |
���� ��1�����װ��������ԣ������ᷴӦ���ɣ�CH3��2NH2Cl��[��CH3��2NH22+]����ˮ����Һ�����ԣ��ݴ��ж���Һ�и�����Ũ�ȴ�С��
��2����Ӧ�Է����е��ж�����Ϊ����H-T��S��0������
��3����2CH3OH?CH3OCH3+H2O����Ӧǰ�����ʵ������䣬����������ͬ�����½��У���Ӧǰ�����ʵ������䣬��ʼ����Ϊ���2���������������ʵ���ҲΪ������
�ڼ����ʱŨ���̣���ƽ�ⳣ���ȽϷ����жϷ�Ӧ���з���
������ͼ�� ��ƽ�ⳣ�������������м������ʵ�������������ȽϷ����¶ȱ仯���������ʵ����ı仯������
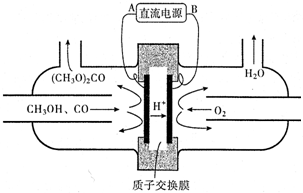
��4��ͼ������֪��������������������˵缫���ӵ�Ϊ�������������Ǽ״���һ����̼��Ӧʧ���ӷ���������Ӧ�����ݵ����غ���㣮
��� �⣺��1�����װ��������ԣ������ᷴӦ���ɣ�CH3��2NH2Cl��[��CH3��2NH22+]����ˮ����Һ�����ԣ��ݴ��ж���Һ�и�����Ũ�ȴ�СΪ��c��Cl-����c[��CH3��2NH2+]��c��H+����c��OH-����
�ʴ�Ϊ��c��Cl-����c[��CH3��2NH2+]��c��H+����c��OH-����
��2���״��ϳɶ����������Ļ�ѧ����ʽΪ��2CH3OH+NH3+CO?��CH3��2NCHO+2H2O���÷�Ӧ�ڳ��������Է����У���S��0����H-T��S��0���H��0��
�ʴ�Ϊ������
��3����2CH3OH?CH3OCH3+H2O����Ӧǰ�����ʵ������䣬����������ͬ�����½��У���Ӧǰ�����ʵ������䣬��ʼ����Ϊ���2���������������ʵ���ҲΪ����������x��y=1��2��
�ʴ�Ϊ��$\frac{1}{2}$��
����֪387��ʱ�÷�Ӧ�Ļ�ѧƽ�ⳣ��K=4������ʼʱ������I�г���0.1mol CH3OH��0.15mol CH3OCH3��0.10mol H2O��Ũ����Qc=$\frac{0.15��0.1}{0��{1}^{2}}$=1.5��K=4����Ӧ��������Ӧ���У�
�ʴ�Ϊ������
�ۢ��� �����ɼ���Ϊ���ʵ���x
2CH3OH?CH3OCH3+H2O
��ʼ����mol�� 0.2 0 0
�仯����mol�� 2x x x
ƽ������mol��0.2-2x x x
K=$\frac{{x}^{2}}{��0.2-2x��^{2}}$=4
x=0.08mol��
�͢�Ƚϣ���ʼ����ͬ���¶Ƚ���ƽ��������У�����ӦΪ���ȷ�Ӧ�������¶�����ѵIJ��ʣ�
�ʴ�Ϊ�����£�
��4��ͼ������֪��������������������˵缫���ӵ�B�缫Ϊ�������������Ǽ״���һ����̼��Ӧʧ���ӷ���������Ӧ���缫��ӦΪ��2CH3OH+CO-2e-=��CH3O��2CO+2H+�����μӷ�Ӧ��O2Ϊ1.12m3����״���������ʵ���=$\frac{1120L}{22.4L/mol}$=50mol������ת��50mol��4=200mol�����Ƶ�̼�������������=100mol��90g/mol=9000g=9.00kg��
�ʴ�Ϊ��B��2CH3OH+CO-2e-=��CH3O��2CO+2H+��9.00��
���� ���⿼��������ˮ����������ԭ���ķ������㣬��ѧƽ���ƽ�ⳣ������Ӧ�ã����ռ״��ǹؼ�����Ŀ�Ѷ��еȣ�


 Сѧ�̲�ȫ��ϵ�д�
Сѧ�̲�ȫ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �ӻ�ǰ��Ĺ�������䣬���������״�����˸ı� | |
| B�� | sp3��sp2��sp�ӻ�����ļнǷֱ�Ϊ109��28�䡢120�㡢180�� | |
| C�� | �����������Ρ������Ρ�V�η��ӵĽṹ������sp3�ӻ�������� | |
| D�� | �ӻ����ȫ���μ��γɻ�ѧ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
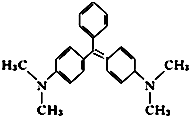 ��ȸʯ���ǻ�����Ʒ�����нϸ߶��ԣ��߲����������°����»��� ��ṹ��ʽ��ͼ��ʾ�����й��ڿ�ȸʯ�̵�˵����ȷ���ǣ�������
��ȸʯ���ǻ�����Ʒ�����нϸ߶��ԣ��߲����������°����»��� ��ṹ��ʽ��ͼ��ʾ�����й��ڿ�ȸʯ�̵�˵����ȷ���ǣ�������| A�� | ��ȸʯ�̵ķ���ʽΪC23H25N2 | |
| B�� | ��ȸʯ�����ڷ����� | |
| C�� | ��ȸʯ�̱����ϵ�һ��ȡ������5�� | |
| D�� | 1mol��ȸʯ����һ��������������6 mol H2�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
| A�� | ��Ӧ��ɺ�O2��ʣ�� | |
| B�� | ԭ��������У�C2H4��C2H2�������Ϊ1.9L | |
| C�� | ��Ӧ��ɺ�����ˮ������Ϊ9g | |
| D�� | ԭ��������У�CO��CH4�������һ��Ϊ1��1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
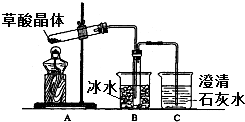 ���ᣨ�Ҷ��ᣩ��������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5����������κͼ���������ˮ���������������ˮ�����ᾧ�壨H2C2O4•2H2O����ɫ���۵�Ϊ101�棬������ˮ��������ˮ��������170�����Ϸֽ⣮�ش��������⣺
���ᣨ�Ҷ��ᣩ��������Ȼ���ֲ���У���K1=5.4��10-2��K2=5.4��10-5����������κͼ���������ˮ���������������ˮ�����ᾧ�壨H2C2O4•2H2O����ɫ���۵�Ϊ101�棬������ˮ��������ˮ��������170�����Ϸֽ⣮�ش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
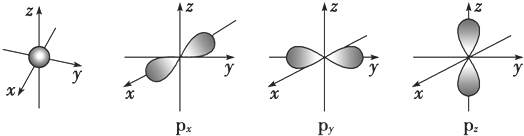
 ����Ԫ��X��ԭ�����������Ų�ʽΪnsn-1npn+1����ôX��Ԫ�ط���ΪS��ԭ�ӵĵ����Ų�ͼΪ
����Ԫ��X��ԭ�����������Ų�ʽΪnsn-1npn+1����ôX��Ԫ�ط���ΪS��ԭ�ӵĵ����Ų�ͼΪ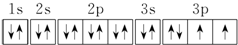 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | Na��Mg��Al��ԭ�����μ��� | B�� | I2��Br2��Cl2������������ǿ | ||
| C�� | C��N��Oԭ�Ӱ뾶���μ�С | D�� | P��S��Cl����������ν��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������Һ��������ˮ���ٻָ���20��ʱ����Һ�ܶ�һ������1.174 g•cm-3 | |
| B�� | ����Һ��KCl����������Ϊ$\frac{74.5}{1.174��1000}$��100% | |
| C�� | �ܶȴ���1.174 g•cm-3��KCl��Һ�ǹ�������Һ | |
| D�� | 25��ʱ������KCl��Һ��Ũ�ȴ���4.0 mol•L-1 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com