
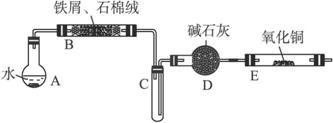
�ٽ�5% Na2CO3��Һ���뵽ʢ��һ��������м���ձ��У����������ӣ�ȥ��Na2CO3��Һ��Ȼ����м������ˮϴ��2��3�飬����ֽ����ˮ�֡�
�ڽ�ϴ�Ӹ����ķ���м��ʯ������������������õ�����װ��B�С�
���ȵ�ȼX���ľƾ��ƣ����Թ�C�г��ִ���ˮ��ʱ����ȼY���ľƾ���ơ�
�ܾ�����Ҫ��ʵ�������ȼZ���ľƾ��ơ�
��ֹͣ��Ӧ����B����ȴ��ȡ���еĹ��壬�������ϡ�����ַ�Ӧ�����ˡ�
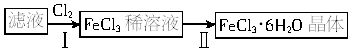
������������Һ��ȡFeCl3��6H2O���壬����������£�
Һ��![]() FeCl3��Һ
FeCl3��Һ![]() FeCl3��6H2O����
FeCl3��6H2O����
�ش��������⣺
��1��ʵ�鲽��ٵ�Ŀ����________________________________________________________��
��2�������X����ָ___________(��ͼ�е�A��B��C������ʾ�������Թ�C�г��ִ���ˮ��ʱ�ŵ�ȼY���ľƾ���ƣ���������Ŀ���ǣ�______________________________________��
��3������ܱ�Ҫ��ʵ�������ָ��________________________________________________��
��4�������FeCl3��Һ�õ�FeCl3��6H2O�������Ҫ����������______________________������������豣�������������Ҫԭ����___________________________________________��
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
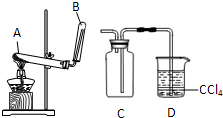 ijУ��ѧС���ͬѧ������ͼ��ʾװ����ʵ��������ȡ������
ijУ��ѧС���ͬѧ������ͼ��ʾװ����ʵ��������ȡ������
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
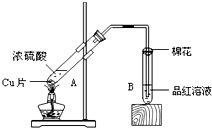
| B������� | �� | �� | �� |
| ��պ�Լ� | ���з�̪��NaOH��Һ | Ʒ����Һ | ���ۺ͵�ˮ���Һ |
| ���� | ��Һ��ɫ��ʧ ��Һ��ɫ��ʧ |
Ʒ����ɫ Ʒ����ɫ |
��ɫ��ɫ ��ɫ��ɫ |
| ����SO2������ | ���� ���� |
Ư���� Ư���� |
��ԭ�� ��ԭ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ijУ��ѧС���ͬѧ��������װ�ý��С�����ˮ��Ӧ����ʵ�飬�����ò����һ����ȡFeCl3?6H2O���塣��ͼ�мгּ�β������װ�þ���ȥ��
ijУ��ѧС���ͬѧ��������װ�ý��С�����ˮ��Ӧ����ʵ�飬�����ò����һ����ȡFeCl3?6H2O���塣��ͼ�мгּ�β������װ�þ���ȥ��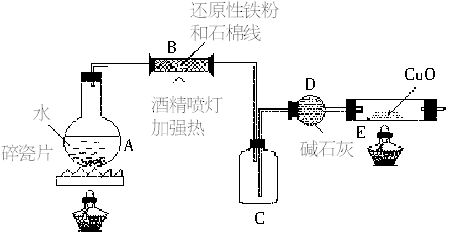
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com