
 2SO3��g�����ô�ѭ��������V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2����Ϊ��������������Ӧ�Ļ�ѧ����ʽΪ��SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5��
2SO3��g�����ô�ѭ��������V2O5����SO2ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2����Ϊ��������������Ӧ�Ļ�ѧ����ʽΪ��SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5�� 2SO3��g����SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5��
2SO3��g����SO2+V2O5=SO3+2VO2�� O2+4VO2=2V2O5��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ||
| �� |
| ||
| �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
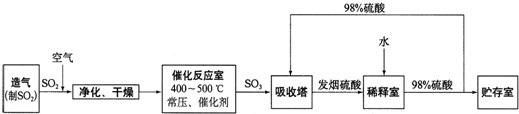
����ѧ����ѧ�뼼������12�֣����Ṥҵ����������ʾ��
��1������Ӧ�ҷ�����Ӧ�Ļ�ѧ����ʽ�ǣ� ���÷�Ӧͨ���� V2O5 ��������������������ǣ�V2O5 ����SO2 ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2 ������д���ô�ѭ�������Ļ�ѧ����ʽ��
��
��2����������ͼ�ж�����˵����ȷ���� ������ĸ����
a��������������� SO2 ��ת����
b��ʹ�ô�������� SO2 �ķ�Ӧ���ʺ�ת����
c���� 98%���������� SO3 �����Ա����γ����������������
��3��ÿ160g SO3 ������ H2O(l) ���Ϸų� 260.6 kJ ���������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�������������˷�ֹSO2��Ⱦ�������õ�����⣬��ҪĿ���ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ��Ϫ�и߶���ѧ����ĩ���Ի�ѧ���������� ���ͣ������
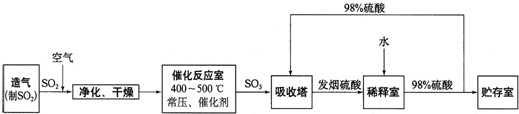
����ѧ����ѧ�뼼������12�֣����Ṥҵ����������ʾ��
��1������Ӧ�ҷ�����Ӧ�Ļ�ѧ����ʽ�ǣ� ���÷�Ӧͨ����V2O5 ��������������������ǣ�V2O5 ����SO2 ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ�O2 ������д���ô�ѭ�������Ļ�ѧ����ʽ��
��
��2����������ͼ�ж�����˵����ȷ���� ������ĸ����
a���������������SO2 ��ת����
b��ʹ�ô��������SO2 �ķ�Ӧ���ʺ�ת����
c����98%����������SO3 �����Ա����γ����������������
��3��ÿ160g SO3 ������H2O(l)���Ϸų�260.6kJ���������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�������������˷�ֹSO2 ��Ⱦ�������õ�����⣬��ҪĿ���ǣ� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010������ʡ��Ϫ�и߶���ѧ����ĩ���Ի�ѧ���������� ���ͣ������
����ѧ����ѧ�뼼������12�֣����Ṥҵ����������ʾ��
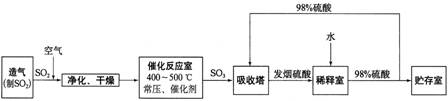
��1������Ӧ�ҷ�����Ӧ�Ļ�ѧ����ʽ�ǣ� ���÷�Ӧͨ���� V2O5 ��������������������ǣ�V2O5 ���� SO2 ʱ����������ԭΪ�ļ۷�������ļ۷��������ٱ� O2 ������д���ô�ѭ�������Ļ�ѧ����ʽ��
��
��2����������ͼ�ж�����˵����ȷ���� ������ĸ����
a��������������� SO2 ��ת����
b��ʹ�ô�������� SO2 �ķ�Ӧ���ʺ�ת����
c���� 98%���������� SO3 �����Ա����γ����������������
��3��ÿ160g SO3 ������ H2O (l) ���Ϸų� 260.6 kJ ���������÷�Ӧ���Ȼ�ѧ����ʽ�� ��
��4���������ų���β�����ð�ˮ���գ�����Ũ���ᴦ�������������˷�ֹSO2 ��Ⱦ�������õ�����⣬��ҪĿ���ǣ� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com