
��10�֣���һ����4�֣�д���йط�Ӧ�����ӷ���ʽ�� ��1������������Һ��̼��������Һ�ķ�Ӧ �� ��2��������Ͷ��ϡ�����з�Ӧ ��
��3����Na2CO3��Һ�м�������ϡ���� ��
��4����̼�������Һ�м��������ռ���Һ�������������������������������������� ��
������(6��)ij��ɫ��Һ����Na����Ag����Ba2����Al3����AlO��MnO��CO��SO�е���������ɣ�ȡ����Һ��������ʵ�飺
��ȡ������Һ������������ᣬ���������ɣ����õ���Һ��
���ڢ�������Һ���ټ������̼�������Һ�����������ɣ�ͬʱ������ɫ�����ף�
���ڢ�������Һ�м������Ba(OH)2��Һ��Ҳ���������ɣ����а�ɫ������������
��������ʵ��ش��������⣺
(1)��Һ��һ�������ڵ�������___________________________________________��
(2)һ�����ڵ�������___________________________________________________��
(3)�жϳ����ҳɷֵķ����� ��
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| O | - 2 |
| O | - 4 |
| O | 2- 3 |
| O | 2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���һ����4�֣�д���йط�Ӧ�����ӷ���ʽ�� ��1������������Һ��̼��������Һ�ķ�Ӧ �� ��2��������Ͷ��ϡ�����з�Ӧ ��
��3����Na2CO3��Һ�м�������ϡ���� ��
��4����̼�������Һ�м��������ռ���Һ�������������������������������������� ��
������(6��)ij��ɫ��Һ����Na����Ag����Ba2����Al3����AlO��MnO��CO��SO�е���������ɣ�ȡ����Һ��������ʵ�飺
��ȡ������Һ������������ᣬ���������ɣ����õ���Һ��
���ڢ�������Һ���ټ������̼�������Һ�����������ɣ�ͬʱ������ɫ�����ף�
���ڢ�������Һ�м������Ba(OH)2��Һ��Ҳ���������ɣ����а�ɫ������������
��������ʵ��ش��������⣺
(1)��Һ��һ�������ڵ�������___________________________________________��
(2)һ�����ڵ�������___________________________________________________��
(3)�жϳ����ҳɷֵķ����� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
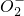 ��Mn
��Mn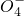 ��C
��C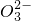 ��S
��S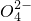 �е���������ɣ�ȡ����Һ��������ʵ�飺
�е���������ɣ�ȡ����Һ��������ʵ�飺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| O | -2 |
| O | -4 |
| O | 2-3 |
| O | 2-4 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com