
œ¬Ν–ΗςΉιάκΉ”‘Ύ÷ΗΕ®»ή“Κ÷–Ρή¥σΝΩΙ≤¥φΒΡ « Θ® Θ©
ΔΌΈό…Ϊ»ή“Κ÷–ΘΚK+ΓΔNa+ΓΔCr2 072®CΓΔS04 2-
ΔΎpH =11ΒΡ»ή“Κ÷–ΘΚCO32-ΓΔNa+ΓΔN03-ΓΔAlO2-
ΔέΦ”»κAlΡήΖ≈≥ωH2ΒΡ»ή“Κ÷–ΘΚCl-ΓΔS042 -ΓΔN03®CΓΔMg2+
Δή‘Ύ”…Υ°Βγάκ≥ωΒΡcΘ®OH-Θ©= 10®C3ΒΡ»ή“Κ÷–ΘΚNa+ΓΔBa2+ΓΔCl-ΓΔI-
Δί‘Ύ»ή“Κ÷–Ρή¥σΝΩΙ≤¥φΘ§Φ”»κNaOHΚσΦ”»»Φ»”–ΤχΧεΖ≈≥ω”÷”–≥ΝΒμ…ζ≥…ΒΡΘΚCa2+ΓΔHC03®CΓΔNH4+ΓΔAlO2®C
ΔόΡή ΙΈό…ΪΖ”ΧΣ±δΚλ…ΪΒΡ»ή“ΚΘΚNa+ΓΔ Cl -ΓΔS2-ΓΔS032-
AΘ°ΔΌΔέΔί BΘ°ΔΎΔήΔί CΘ°ΔΎΔήΔό DΘ°ΔήΔίΔό
 ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ
ΧλΧλœρ…œ“Μ±ΨΚΟΨμœΒΝ–¥πΑΗ –Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
–Γ―ß…ζ10Ζ÷÷””Π”ΟΧβœΒΝ–¥πΑΗ
| ΡξΦΕ | ΗΏ÷–ΩΈ≥Χ | ΡξΦΕ | ≥θ÷–ΩΈ≥Χ |
| ΗΏ“Μ | ΗΏ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ“Μ | ≥θ“ΜΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏΕΰ | ΗΏΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θΕΰ | ≥θΕΰΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
| ΗΏ»ΐ | ΗΏ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ | ≥θ»ΐ | ≥θ»ΐΟβΖ―ΩΈ≥ΧΆΤΦωΘΓ |
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ2013-2014―ßΡξΚΰΡœ Γ≥Λ…≥ –ΗΏ»ΐΒΎΕΰ¥Έ‘¬ΩΦΜ·―ß ‘ΨμΘ®ΫβΈωΑφΘ© Χβ–ΆΘΚ―Γ‘ώΧβ
œ¬Ν–ΗςΉιάκΉ”‘Ύ÷ΗΕ®»ή“Κ÷–Θ§Ω…Ρή¥σΝΩΙ≤¥φΒΡ «Θ® Θ©
ΔΌ»ή”–ΉψΝΩΑ±ΤχΒΡΈό…Ϊ»ή“Κ÷–ΘΚK+Θ§NH Θ§Na+Θ§HSO
Θ§Na+Θ§HSO Θ§PO
Θ§PO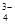 Θ§MnO
Θ§MnO
ΔΎΡή ΙpH ‘÷Ϋ±δ…νάΕ…ΪΒΡ»ή“Κ÷–ΘΚK+Θ§Na+Θ§AlO Θ§NO
Θ§NO Θ§S
Θ§S Θ§SO
Θ§SO
ΔέΥ°ΒγάκΒΡH+≈®Ε»c(H+)=10-13molΓΛL-1ΒΡ»ή“Κ÷–ΘΚCl Θ§CO
Θ§CO Θ§S2O
Θ§S2O Θ§Fe3+Θ§K+Θ§Na+
Θ§Fe3+Θ§K+Θ§Na+
ΔήΦ”»κAlΡήΖ≈≥ωH2ΒΡ»ή“Κ÷–ΘΚNa+Θ§NH Θ§K+Θ§SO
Θ§K+Θ§SO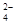 Θ§Cl
Θ§Cl Θ§Br
Θ§Br
Δί Ι ·»ο±δΚλΒΡ»ή“Κ÷–ΘΚFe3+Θ§Na+Θ§K+Θ§NO Θ§SO
Θ§SO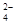 Θ§Cl
Θ§Cl
Δό “Έ¬œ¬œ¬pH=1ΒΡ»ή“Κ÷–ΘΚFe2+Θ§Al3+Θ§K+Θ§Na+Θ§NO Θ§I
Θ§I Θ§Cl
Θ§Cl Θ§S
Θ§S
ΔΏΚ§”–¥σΝΩFe3+ΒΡ»ή“ΚΘΚK+Θ§NH Θ§Na+Θ§SCN
Θ§Na+Θ§SCN Θ§I
Θ§I Θ§Br
Θ§Br
Δύ “Έ¬œ¬Θ§ =0.1 mol/LΒΡ»ή“Κ÷–ΘΚNa+Θ§K+Θ§SO
=0.1 mol/LΒΡ»ή“Κ÷–ΘΚNa+Θ§K+Θ§SO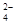 Θ§SiO
Θ§SiO Θ§NO
Θ§NO
AΘ°ΔΌΔέΔήΔό BΘ°ΔΌΔέΔόΔΏ CΘ°ΔΎΔήΔίΔύ DΘ°ΔΎΔίΔΏΔύ
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΩΤΡΩΘΚΗΏ÷–Μ·―ß ά¥‘¥ΘΚ Χβ–ΆΘΚ
‘Ύ÷ΗΕ®ΜΖΨ≥÷–Θ§œ¬Ν–ΗςΉιάκΉ”“ΜΕ®Ω…“‘¥σΝΩΙ≤¥φΒΡ ( )
A. ΙpH ‘÷Ϋ≥ Κλ…ΪΒΡ»ή“Κ: ![]()
B.≥ΘΈ¬œ¬Θ§‘Ύ![]() ΒΡ»ή“¥:
ΒΡ»ή“¥:![]()
C.Φ”»κ¬ΝΖέΖ≈≥ω«βΤχΒΡ»ή“ΚΘΚ![]()
D. ΙΈό…ΪΖ”ΧΣ ‘“Κœ‘Κλ…ΪΒΡ»ή“ΚΘΚ![]()
≤ιΩ¥¥πΑΗΚΆΫβΈω>>
ΙζΦ ―ß–Θ”≈―Γ - ΝΖœΑ≤αΝ–±μ - ‘ΧβΝ–±μ
Κΰ±± ΓΜΞΝΣΆχΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΤΫΧ® | Άχ…œ”–ΚΠ–≈œΔΨΌ±®Ή®«χ | Βγ–≈’©Τ≠ΨΌ±®Ή®«χ | …φάζ Ζ–ιΈό÷ς“ε”–ΚΠ–≈œΔΨΌ±®Ή®«χ | …φΤσ«÷»®ΨΌ±®Ή®«χ
ΈΞΖ®ΚΆ≤ΜΝΦ–≈œΔΨΌ±®ΒγΜΑΘΚ027-86699610 ΨΌ±®” œδΘΚ58377363@163.com