
������������ʽ����ƽ�������(N2H4)��Ϊȼ�ϣ�NO2��Ϊ����������Ӧ����N2��ˮ��������֪��N2(g) + 2O2(g) ��2NO2(g)�� ��H����67.7 kJ/mol
N2H4(g) + O2(g) �� N2(g) + 2H2O(g)�� ��H����534 kJ/mol
���й����º�NO2��Ӧ���Ȼ�ѧ����ʽ�У���ȷ����
| A��2N2H4(g) + 2NO2(g) �� 3N2(g) + 4H2O(l)����H����1135.7 kJ/mol |
| B��N2H4(g) + NO2(g) �� 3/2N2(g) + 2H2O(g)����H����567.85 kJ/mol |
| C��N2H4(g) + NO2(g) �� 3/2N2(g) + 2H2O(l)����H����1135.7 kJ/mol |
| D��2N2H4(g) + 2NO2(g) = 3N2(g) + 4H2O(g)����H��+1135.7 kJ/mol |
 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ���꣬�ҹ��ں�����ҵ��ȡ������������Ŀ�ijɾͣ����۷ɴ���α�����ϵ�л������̫�գ�
���꣬�ҹ��ں�����ҵ��ȡ������������Ŀ�ijɾͣ����۷ɴ���α�����ϵ�л������̫�գ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
N2H4(g)+O2(g)====N2(g)+2H2O(g);��H=-534 kJ��mol-1
���й����º�NO2��Ӧ���Ȼ�ѧ����ʽ�У���ȷ����
A.2N2H4��g��+2NO2(g) ====3N2(g)+4H2O(l);��H=-1 135.7 kJ��mol-1
B.2N2H4(g)+2NO2(g) ====3N2(g)+4H2O(g);��H=-1 000.3 kJ��mol-1
C.N2H4(g)+NO2(g) ====3/2N2(g)+2H2O(l);��H=-1 135.7 kJ��mol-1
D.2N2H4(g)+2NO2(g) ====3N2(g)+4H2O(g);��H=-1 135.7 kJ��mol-1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�����и��������ε��п��ԣ���ѧ���⣩ ���ͣ������
���꣬�ҹ��ں�����ҵ��ȡ������������Ŀ�ijɾͣ����۷ɴ���α�����ϵ
�л������̫�ա�
(1)������������ʽ����ƽ���������(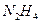 )��Ϊȼ�ϣ�
)��Ϊȼ�ϣ� ��Ϊ�ƽ�����
��Ϊ�ƽ�����
�� ����Ҫ��������ȼ�������ڹ���ʱ���������ɫ����
����Ҫ��������ȼ�������ڹ���ʱ���������ɫ����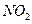 ���Ի�������
���Ի�������
����Ⱦ��Ϊ������Ⱦ��ʹ������ (����ĸ)����֮��
| A��Һ̬�� | B��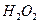 | C��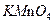 | D��Һ̬�� |
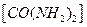 �ʹ������Ʒ�Ӧ������ȡ����(������
�ʹ������Ʒ�Ӧ������ȡ����(������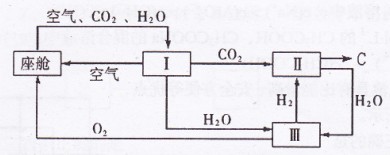
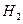 ��
��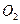 �� ��װ�â���
�� ��װ�â���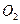 ����Դ�����Աÿ������35mol
����Դ�����Աÿ������35mol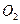 ��ÿ�����
��ÿ�����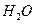 ��������������к�
��������������к�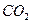 mol��
mol���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ�긣��ʡ�����и�����ѧ��12���¿���ѧ�Ծ� ���ͣ�ѡ����
������������ʽ����ƽ�������(N2H4)��Ϊȼ�ϣ�NO2��Ϊ����������Ӧ����N2��ˮ��������֪��N2(g) + 2O2(g) ��2NO2(g)�� ��H����67.7 kJ/mol
N2H4(g) + O2(g) �� N2(g) + 2H2O(g)�� ��H����534 kJ/mol
���й����º�NO2��Ӧ���Ȼ�ѧ����ʽ�У���ȷ����
A��2N2H4(g) + 2NO2(g) �� 3N2(g) + 4H2O(l)����H����1135.7 kJ/mol
B��N2H4(g) + NO2(g) �� 3/2N2(g) + 2H2O(g)����H����567.85 kJ/mol
C��N2H4(g) + NO2(g) �� 3/2N2(g) + 2H2O(l)����H����1135.7 kJ/mol
D��2N2H4(g) + 2NO2(g) = 3N2(g) + 4H2O(g)�� ��H��+1135.7 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���꣬�ҹ��ں�����ҵ��ȡ������������Ŀ�ijɾͣ����۷ɴ���α�����ϵ
�л������̫�ա�
(1)������������ʽ����ƽ���������(![]() )��Ϊȼ�ϣ�
)��Ϊȼ�ϣ�![]() ��Ϊ�ƽ�����
��Ϊ�ƽ�����
��![]() ����Ҫ��������ȼ�������ڹ���ʱ���������ɫ����
����Ҫ��������ȼ�������ڹ���ʱ���������ɫ����![]() ���Ի�������
���Ի�������
����Ⱦ��Ϊ������Ⱦ��ʹ������ (����ĸ)����֮��
A��Һ̬�� B��![]() C��
C��![]() D��Һ̬��
D��Һ̬��
���ڼ��������£�������![]() �ʹ������Ʒ�Ӧ������ȡ����(������
�ʹ������Ʒ�Ӧ������ȡ����(������
ͬʱ����������)����÷�Ӧ�����ӷ���ʽ�� ��
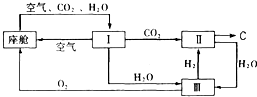
(2)�ɴ������ڿ����ĸ��¹�������ͼ��ʾ��
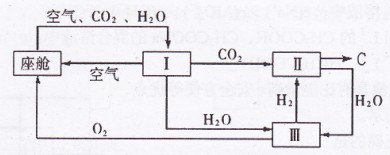
�������ڿ������¹��̿���ѭ�����õ�����Ϊ![]() ��
��![]() �� ��װ�â���
�� ��װ�â���
������Ӧ�Ļ�ѧ����ʽΪ ��
�ڴ�װ��I����ɿ���![]() ����Դ�����Աÿ������35mol
����Դ�����Աÿ������35mol![]() ��ÿ�����
��ÿ�����
�������к�18 mol![]() ��������������к�
��������������к�![]() mol��
mol��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com