
 ��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д�
��ְٷְټ�����Ԫ��ĩ���Ծ�ϵ�д� Сѧ��ĩ���Ծ�ϵ�д�
Сѧ��ĩ���Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ2007����ѧ�ڸ����꼶�������Ͽ��ԡ���ѧ���� ���ͣ�058
| |||||||||||||||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꼪��ʡ������ѧ�ڵڶ��������Ի�ѧ�� ���ͣ������
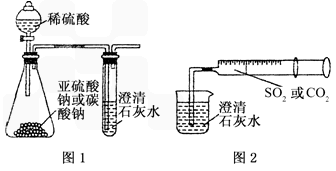
��12�֣�ijͬѧ����ͼһ��ʾ��װ����̽��CO2��SO2�����ʯ��ˮ�ķ�Ӧ�����ͨ��CO2���Կ����Ȼ��Ǻ���������ͨ��SO2û���ܿ�������������˼��������ͬѧ����ͼ����װ�ã��������ռ���ע�����������ؽ�����һ������һ�����ݵ�ͨ�����ʯ��ˮ�У����ܿ���ʯ��ˮ�ȱ�����ٳ����������ͨ��SO2�����������Ա�ͨ��CO2�졣
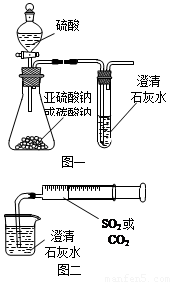
��1���Աȷ�������ʵ�飬����Ϊ��ͼһװ��ʵ��
ʱ��ͨ��SO2���ܳ��ֻ��ǵ�ԭ������ǣ�
_________________��
��2����ͼ��װ��ʵ��ʱ������ͬ����ͨ��CO2��
SO2��SO2�������ǡ�����������CO2
���ԭ����______________________________________��
��3����ͼһ����SO2��ʯ��ˮ��Ӧ��ʵ��ʱ���Ӱ�ȫ�Ƕ�
����װ��Ӧ���θĽ���
_____________________________________________��
��4�������������ʵ������ʯ��ˮ�ȱ�����ٳ��塱���������ʯ��ˮ��Ũ���йء�Ϊ��̽��CO2ͨ�����ʯ��ˮ�е�ʵ��������������ݣ�
�� 20��ʱ��Ca(OH)2 ���ܽ��Ϊ��0.165g/100gˮ��
�� ��ͬŨ��ʯ��ˮ����CaCO3�������
|
����ʯ��ˮ��ˮ������� |
1:0 |
1:1 |
1:2 |
1:3 |
1:5 |
1:7 |
|
������CaCO3���������g/100ˮ�� |
A |
0.110 |
0.073[��Դ:Zxxk.Com] |
0.055 |
0.037 |
0.028 |
�� �ϱ���A= g/100gˮ
�� ��1.01��105Pa CO2ѹ���£�CaCO3���ܽ��
|
����ѧ�¶�/K |
282 |
298 |
308 |
|
CaCO3�ܽ�ȣ�g/100ˮ��[��Դ:ѧ#��#��Z#X#X#K] |
0.130 |
0.094 |
0.076 5 |
�� �ڲ�ͬѹǿ��CO2���£�CaCO3�ܽ�ȣ�18�棩
|
P(CO2)/Pa |
0 |
1.40��104 |
9.95��104 |
|
CaCO3�ܽ�ȣ�g/100ˮ�� |
0.001 3 |
0.023 3 |
0.108 6 |
��������������ݻش��������⣺
���ɱ���ͱ�����֪CaCO3�ܽ�ȵı仯�����ǣ�
�����������ݿ��Եó����ۣ����۲쵽��ʯ��ˮ�ȱ�����ٳ������������Ҫ��ʵ�������ǣ�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com