
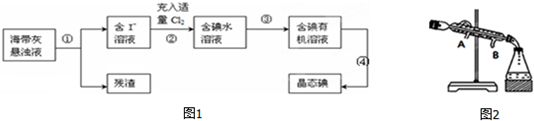
���� ����ȡ���ʵ��ʵ�����̿�֪����Ϊ���ˣ���Һ�к������ӣ����з���Cl2+2I-=I2+2Cl-���õ���ˮ��Һ����Ϊ��ȡ��Һ�õ�������л���Һ����Ϊ���ɵõ���̬�⣬�Դ˽��
��1������װ����������д�����ƣ�
��2�������������������������������ɵ��ʵ⣻
��3���ӵ�ˮ�л�ȡ�ⵥ�ʲ�����ȡ�ķ�������ȡ����ѡȡ���ǣ���������ȡ���е��ܽ�ȴ�����ԭ�ܼ��е��ܽ�ȣ����ʺ���ȡ������Ӧ����ȡ����ԭ���ܼ����ܻ��ܣ����Ȼ�̼������ȡ����ѡȡ�������Կ��������Ȼ�̼����ȡ��������������Һ���˵õ�����-����Һ��ͨ���������������ӵõ���ˮ��Һ���������Ȼ�̼������ȡ�ⵥ�ʣ�����õ��ⵥ�ʣ�
��4����ȡ˳��Ϊ��1����װ����̨��2������Ҫ���������ѡ���ʵ����ܼ�����ȡ������3���Ƚ�Ҫ��������ʵ���Һ�����Һ©���У�Ȼ��ע����ȡ����4�����ã���Һ��ֳ���������������������²�Һ��ų���Ȼ��ر����������ϲ�Һ����Ͽڵ�����5������װ�ã�
��5��ˮ����������Ч���ã�
��� �⣺����ȡ���ʵ��ʵ�����̿�֪����Ϊ���ˣ���Һ�к������ӣ����з���Cl2+2I-=I2+2Cl-���õ���ˮ��Һ����Ϊ��ȡ��Һ�õ�������л���Һ����Ϊ���ɵõ���̬�⣬
��1����Ϊ���ˣ��õ����������ձ���©�����������ȣ��ʴ�Ϊ��©�����ձ�����������
�ʴ�Ϊ��©�����ձ�����������
��2��������������û������ӣ��䷴Ӧ���ӷ���ʽΪCl2+2I-=I2+2Cl-��ʹ������ת��Ϊ�ⵥ�ʣ�
�ʴ�Ϊ��ʹ������ת��Ϊ�ⵥ�ʣ�
��3���ӵ�ˮ�л�ȡ�ⵥ�ʲ�����ȡ�ķ���������������Һ���˵õ�����-����Һ��ͨ���������������ӵõ���ˮ��Һ���������Ȼ�̼������ȡ�ⵥ�ʣ�����õ��ⵥ�ʣ��ƾ��ʹ���������ˮ��������ȡ������ѡBD��
�ʴ�Ϊ����ȡ������ȡ��Һ����BD��
��4����ȡ˳��Ϊ��1����װ����̨��2������Ҫ���������ѡ���ʵ����ܼ�����ȡ������3���Ƚ�Ҫ��������ʵ���Һ�����Һ©���У�Ȼ��ע����ȡ����4�����ã���Һ��ֳ���������������������²�Һ��ų���Ȼ��ر����������ϲ�Һ����Ͽڵ�����5������װ�ã���������ʵ��˳��Ϊ���ߢڢ٢ܢۢޢݣ�
�ʴ�Ϊ��B��
��5������ �ܴӺ�����л���Һ����ȡ�Ⲣ�����л��ܼ�������Ҫ����������������ͼ��������ˮ������ΪB����A��
�ʴ�Ϊ��B����A��
���� ���⿼���˺�ˮ��Դ���ۺ����ã��漰֪ʶ��϶࣬��ȡ����ѡȡ������ȡ�ֲ����������Һ��ķ��뷽�����dz�����㣬��Ҳ���״��㣬�ѶȲ���


 ����ͼ���������������ϵ�д�
����ͼ���������������ϵ�д� ����ѧҵ���Ե�����ϵ�д�
����ѧҵ���Ե�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����

| A�� | ͼ1�У����뺣ˮ�е�������Խ�����˸�ʴԽ���� | |
| B�� | ͼ2�У�һ��ʱ������ְ�ɫ����-����ɫ����-���ɫ���������� | |
| C�� | ͼ3�У�ȼ��������IJ�λ�������⣬��Ҫ�����ڸ�������������ѧ��ʴ | |
| D�� | ͼ4�У�������þ��ķ�������ֹ���¸����ܵ��ĸ�ʴ��þ���൱��ԭ��ص����� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ۢݢ� | B�� | �ڢܢݢ� | C�� | �ڢۢޢ� | D�� | �ۢܢޢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | HCO3-+H2O?CO2��+H2O+OH- | B�� | HS-+H2O?H3O++S2- | ||
| C�� | Fe3++3H2O?Fe��OH��3��+3H+ | D�� | CO32-+H2O?HCO3-+OH- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 �������ƣ�NaClO2����һ�ָ�Ч��������Ư������֪ NaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2•3H2O������38��ʱ������������ˮNaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ���Ʊ��������ƣ�
�������ƣ�NaClO2����һ�ָ�Ч��������Ư������֪ NaClO2������Һ���¶ȵ���38��ʱ�����ľ�����NaClO2•3H2O������38��ʱ������������ˮNaClO2������60��ʱNaClO2�ֽ��NaClO3��NaCl��������ͼ��ʾװ���Ʊ��������ƣ�| ʵ����� | �ζ�ǰ����/mL | �ζ������/mL |
| 1 | 0.00 | 19.96 |
| 2 | 3.26 | 23.30 |
| 3 | 1.10 | 23.40 |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com