
��9�֣���ͼ��ʾ����һ��С�ձ��������ϸ��20g Ba��OH��2��8H2O���塣����С�ձ��������ȵ���3��4��ˮ�IJ���Ƭ�ϣ�Ȼ����С�ձ��м���10g NH4CL���壬���ò��������ٽ��衣
��1��ʵ����Ҫ�ò����������ԭ���� ��
��2��д���÷�Ӧ�Ļ�ѧ����ʽ ��
�÷�Ӧ ����ǡ����ǡ���������ԭ��Ӧ��
��3����ʵ��������� ��
��4���÷�ӦΪ ��������š����ȷ�Ӧ���������ڷ�Ӧ��������� ������ڡ���С�ڡ������������������
��1���÷�Ӧ�ǹ���֮��ķ�Ӧ�������ʹ������ֽӴ���������Ӧ
��2��Ba(OH)2��8H2O+2NH4Cl��BaCl2+2NH3��+10H2O ����
��3������Ƭ����������С�ձ�ճ��һ��ͬʱ�д̼�����ζ���������
��4���� ��
����:��1����Ϊ��Ӧ�ﶼ�ǹ��壬�����ǹ����ķ�Ӧ������ͨ���������Ľ����ʹ������ֽӴ���������Ӧ��
(2)�ڷ�Ӧ��Ԫ�صĻ��ϼ۲�û�з����仯�����Բ���������ԭ��Ӧ��
��3����Ϊ��Ӧ�����ȷ�Ӧ�����Իᵼ���¶Ƚ��ͣ������������ͬʱ�а����ͷų��������Ի����ŵ��д̼�����ζ�����������
��4������лл��֪��Ӧ�����ȵģ���˵����Ӧ���������С�����������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
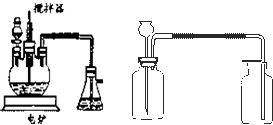 ����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
����ѧ��ѧʵ���У�ͨ������ˮ����ͭ��������ˮ�Ĵ��ڣ�������ˮ����ͭ��ʪ�Ժ�ǿ����Ҫ�������ã�
| ||
| ||
| ||
| ||


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
�鿴�𰸺ͽ���>>
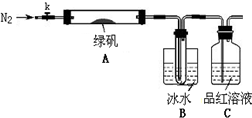
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��ɽ��ʡ������ѧ��ģ�⿼�ԣ����ۣ���ѧ���� ���ͣ�ʵ����
��15�֣�������ѩ����������һ��Сֽ��������д�š����������Ҫ�ɷ�����ʯ�ҡ���Ϊ���ҶԸ�������й�����������̽����
��1������1��Ϊʲô��ʯ�ң�CaO����������������û�ѧ����ʽ��ʾ�� ��
��2�����ֶ�������һϵ��̽������ý϶����棬���й�ʵ�鷽�����£�
|
��������� |
ʵ�鲽�� |
ʵ������ |
ʵ����� |
|
����2��Сֽ���е����� �ܷ������������� |
ȡ����Сֽ���й�������ձ��У���������ˮ���������ڡ� |
|
������ ����� |
|
����3���Ҳ�����ʺ�����ʿ�����̼��ƣ��������֤�ҵIJ��룿 |
|
|
�ø������Ʒ����̼��� |
��3������4����Ʒ��̼��Ƶĺ�����Σ�ijͬѧ���������̽���ʵ�飺�������ܽ�ø������Ʒ�����������������NaOH��Һ�������������NaOH��Һ�����ؼ������Ʒ�ĺ�����ʵ���������ȡ�ø������Ʒ������Ϊ10.0g��
ʵ��װ����ͼ��ʾ��
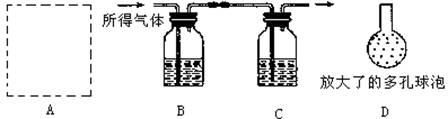 .
.
��AΪ�ܽ�ø������Ʒ��װ�ã��������˳���©����˫�������������ܣ�Ϊ����ܽ����ٻ���Ҫ��ʵ�������� ��
��A�з�����Ӧ�Ļ�ѧ����ʽΪ
�۽�������ҺC�й��ӵ��¶˸ijɾ��ж������(��ͼ�е�D)�����������ʵ���ȷ�ȣ��������� ��
�ܵ��Ľ�ʵ��װ�ò�������ȷ���������ȷ�ⶨ���������Ʒ�ĺ�����������������û����ʧ������ȫ���գ�������ʱCװ����ʵ��ǰ��������������3.6g����ø������Ʒ�ĺ���Ϊ ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0103 ��ĩ�� ���ͣ���ѡ��

[ ]
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��ʾ����һֻʢ��Ba(OH)2��Һ���ձ��У�Ư����һ��Сľ�飬��С�ĵ����ձ��еμ��� Ba(OH)2��Һ�ܶ���ͬ��ϡ���ᣬ��ֹƬ�̺�Сľ�����Һ���е������ԭ�����
A ���� B ����
C ��С D ���ж�
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com