
��ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
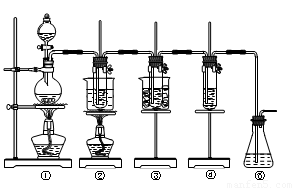
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ��
��Һ����Ϊβ������װ�á�
����д���пհף�
�� ��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��_____________����д�������ƣ�����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е�HCl���壬���ڢ����֮�䰲װʢ��_______����д���б����ĸ���ľ���װ�á�
A.��ʯ�� B.����ʳ��ˮ C.Ũ����
�� �������������������20 mL 12 mol��L��1�������ϼ��ȣ���ַ�Ӧ�����ɵ�������������0.06 mol������Ҫԭ��
��_______________________________________________��
��__________________________________________________________________________��
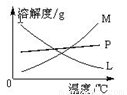
�� �Ƚ���ȡ����غʹ������Ƶ����������ߵIJ�����
�� ��
�� ��
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ
�з��ϸþ����ܽ�����ߵ���_______����д�����ĸ�����Ӣڵ��Թ��з�����þ���ķ�����__________����дʵ��������ƣ���
�� ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհס�
|
ʵ������ |
ԭ�� |
|
��Һ�������ɫ��Ϊ_______ɫ |
������ˮ��Ӧ���ɵ�H+ʹʯ���ɫ |
|
�����Һ��Ϊ��ɫ |
______________________________________ |
|
Ȼ����Һ����ɫ��Ϊ_______ɫ |
______________________________________ |
�ŷ�Һ©�� B
�Ƣ�����ӷ� �ڷ�Ӧһ��ʱ��������ϡ������MnO2����
�Ǣ���ȡ�¶Ȳ�ͬ����KClO3��ϸ��¶ȣ���NaClO�¶Ƚϵͣ�����ȡʱ���Ũ��Ҳ��ͬ����KClO3��Ũ�Ƚϴ�ļ���Һ����NaClO��Ũ�Ƚ�С�ļ���Һ M ����
��
|
ʵ������ |
ԭ�� |
|
��Һ�������ɫ��Ϊ �� ɫ |
������ˮ��Ӧ���ɵ�H+ʹʯ���ɫ |
|
�����Һ��Ϊ��ɫ |
������ˮ��Ӧ����HClO����Ư���� |
|
Ȼ����Һ����ɫ��Ϊdz����ɫ |
���������ܽ���ˮ�� |
����������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����16�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
(1)��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��_____________(��д��������)����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_______(��д���б����ĸ)�ľ���װ�á�
(A)��ʯ�� (B)����ʳ��ˮ (C)Ũ���� (D)����̼��������Һ
(2)�Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����ǣ�____________________________��
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ���_______(��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ�����__________(��дʵ���������)��
(3)��ʵ������ȡ�������Ƶ����ӷ���ʽ��______________________________________��
(4)ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
| ʵ������ | ԭ�� |
| ��Һ�������ɫ��Ϊ_______ɫ | ������ˮ��Ӧ���ɵ�H��ʹʯ���ɫ |
| �����Һ��Ϊ��ɫ | _______________________________ |
| Ȼ����Һ����ɫ��Ϊ_______ɫ | _______________________________ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꼪��ʡ�������и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�ʵ����
��14�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ����������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�����д���пհף�
��1��װ�â�����������װ�ã�Բ����ƿ��ʢ��MnO2���壬�䷴Ӧ�Ļ�ѧ����ʽΪ ��
��2�����ʵ������MnO2�����ˣ����������ʿ��ܿ�����������MnO2��Cl2���ǣ�
A��NaBiO3 B��FeCl3 C��PbO2
����֪������ǿ��˳��Ϊ��NaBiO3��PbO2��MnO2��FeCl3��
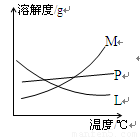
��3���Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����Ǣ� �� �� ��
��4����Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ��� (��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ����� ����дʵ��������ƣ���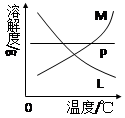
��5��һ������������������ȼ�գ����õĻ������100mL 3.00mol/L��NaOH��Һ���ܶ�Ϊ1.2g/mL��ǡ����ȫ���գ������Һ�к���NaClO�����ʵ���Ϊ0.0500mol��������Һ��Cl�����ӵ����ʵ��� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�����ɹŰ������а���һ�и�����ѧ��12���¿������Ծ�����ѧ���֣� ���ͣ�ʵ����
����16�֣���ʵ�����������ͼ��ʾװ����ȡ����ء��������ƺ�̽����ˮ�����ʡ�
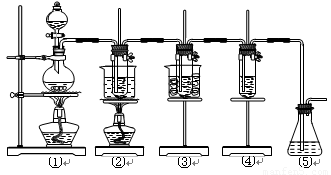
ͼ�У���Ϊ��������װ�ã��ڵ��Թ���ʢ��15 mL 30% KOH��Һ��������ˮԡ�У��۵��Թ���ʢ��15 mL 8% NaOH��Һ�������ڱ�ˮԡ�У��ܵ��Թ��������ɫʯ����Һ����Ϊβ������װ�á�
����д���пհף�
(1)��ȡ����ʱ������ƿ�����һ�����Ķ������̣�ͨ��_____________(��д��������)����ƿ�м���������Ũ���ᡣʵ��ʱΪ�˳�ȥ�����е��Ȼ������壬���ڢ����֮�䰲װʢ��_______(��д���б����ĸ)�ľ���װ�á�
(A)��ʯ�� (B)����ʳ��ˮ (C)Ũ���� (D)����̼��������Һ
(2)�Ƚ���ȡ����غʹ������Ƶ����������ߵIJ����ǣ�____________________________��
��Ӧ��Ͼ���ȴ�ڵ��Թ����д���������������ͼ�з��ϸþ����ܽ�����ߵ���_______(��д�����ĸ)���Ӣڵ��Թ��з�����þ���ķ�����__________(��дʵ���������)��
(3)��ʵ������ȡ�������Ƶ����ӷ���ʽ��______________________________________��
(4)ʵ���пɹ۲쵽�ܵ��Թ�����Һ����ɫ���������±仯������д�±��еĿհף�
|
ʵ������ |
ԭ�� |
|
��Һ�������ɫ��Ϊ_______ɫ |
������ˮ��Ӧ���ɵ�H��ʹʯ���ɫ |
|
�����Һ��Ϊ��ɫ |
_______________________________ |
|
Ȼ����Һ����ɫ��Ϊ_______ɫ |
_______________________________ |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com