
 CO��+ CO2��+ H2O������װ���У�����������ֽ���ȡ������� ������ţ�
CO��+ CO2��+ H2O������װ���У�����������ֽ���ȡ������� ������ţ�

 ͬ����ϰǿ����չϵ�д�
ͬ����ϰǿ����չϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��N2��NO��NH3 | B��NH3��CO2��N2 |
| C��NH3��CO2��NO | D��NH3��N2��NO2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
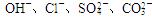 ������϶��ɣ�Ϊ��ȷ�������ֻ�����ijɷ֣�ijͬѧ����������ʵ�������
������϶��ɣ�Ϊ��ȷ�������ֻ�����ijɷ֣�ijͬѧ����������ʵ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��������Һ���Ƿ���CO���μ�ϡ���ᣬ������������ͨ�����ʯ��ˮ |
| B���Ӻ�I������Һ����ȡ�⣺��������ϡ������3%��H2O2��Һ�����þƾ���ȡ |
| C����������ˮ������������Һ�м�������ϡ����ˮԡ���Ⱥ��ټ������Ƶ� Cu��OH��2����Һ������������ |
| D����ȥMg��OH��2�е�����Ca��OH��2������������MgCl2��Һ����ַ�Ӧ�����ϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| | ������Ϊ���� | �����Լ� | ���� |
| 1 | ���飨��ϩ�� | | |
| 2 | �������������ᣩ | | |
| 3 | �������ӣ� | | |
| 4 | �Ҵ���ˮ�� | | |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A������Na2CO3(NaHCO3)������ |
| B��NO(NO2)��ͨ��װˮ��ϴ��ƿ |
| C��NH3(H2O)��ͨ��ʢ��Ũ�����ϴ��ƿ |
| D��Fe2O3(Al2O3)����������������Һ���ٹ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
 �顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ��(��
�顱�õ�һЩ��ʶ��ij��ʦ�������ͼװ��(�� ��װ��������ʡ��)����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a
��װ��������ʡ��)����ʵ�����Ϊ���Ȱ�ͼ��װ�ã��ȹرջ���a��b��c����ͭ˿���м䲿�ּ���Ƭ�̣�Ȼ�����a ��b��c��ͨ�����ƻ���a��b�����н���(��Ъ��)ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺
��b��c��ͨ�����ƻ���a��b�����н���(��Ъ��)ͨ�����壬������M���۲쵽���Ե�ʵ�������Իش��������⣺
 ��Ҫ�ڵ���m��n֮�����Gװ�ã������ӷ�����(��Gװ���е��ܵĴ���)��m��______��_______��n��Gװ�õ������� �������ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թ�F���� �С�
��Ҫ�ڵ���m��n֮�����Gװ�ã������ӷ�����(��Gװ���е��ܵĴ���)��m��______��_______��n��Gװ�õ������� �������ﲻ��ˮ���ն���ֱ����ȴ��Ӧ���Թ�F���� �С��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
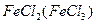 �� ��2��SO2(H2O) ��
�� ��2��SO2(H2O) ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com