
ij��Һ�н����±������е�5������(������ˮ�ĵ��뼰���ӵ�ˮ��)���Ҹ����ӵ����ʵ�����Ϊ0.1mol��
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ������������
������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䣻
������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ�
�Իش��������⣺
��1������ԭ��Һ���ȼ����������ᣬ�ٵ���KSCN��Һ��ʵ��������________��ʵ��������������Ӧ�����ӷ���ʽ��________��
��2��ԭ��Һ��������������(д���ӷ���)________.
��3������ԭ��Һ�м�������NaOH��Һ����ַ�Ӧ����һ��ʱ�䣬���ˡ�ϴ�ӡ����գ��������ù����������________g��


 100�ִ�����ĩ���ϵ�д�
100�ִ�����ĩ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014�콭��ʡ�����и�����ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
|
������ |
SO42-��NO3-��Cl- |
|
������ |
Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣��������Һ�м���BaCl2��Һ���а�ɫ���������Իش��������⡣
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�꽭��ʡ�˴��и���4��ģ�⿼�����ۻ�ѧ�Ծ��������棩 ���ͣ�������
��ij��Һ�н����±������е�5�����ӣ�������ˮ�ĵ��뼰���ӵ�ˮ�⣩�������ӵ����ʵ�����Ϊ1mol��
|
������ |
SO42-��NO3-��Cl- |
|
������ |
Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
������ԭ��Һ�м���KSCN��Һ�������Ա仯��������ԭ��Һ�м�����������ᣬ���������ɣ���Һ������������䡣������ԭ��Һ�м���BaCl2��Һ���а�ɫ�������ɡ��Իش���������
��1��������ԭ��Һ�м�����������ᣬ�ټ���KSCN��Һ�������� ��
��2��ԭ��Һ�к��е��������� ��
��3����ԭ��Һ�м������������ᣬ������Ӧ�����ӷ���ʽΪ ��
��4����ԭ��Һ�м���������NaOH��Һ����ַ�Ӧ���ˡ�ϴ�ӡ����գ��������ù�����������ƽ��������Ϊ ��
��. �����������壨FeC2O4��2H2O����̼��﮺Ͷ�������������и��·�Ӧ���Ʊ�﮵�ص��������Ϲ�������ﮣ�Li2FeSiO4��������������������������н������ط������������ͼ��ʾ��TG%��ʾ������������ռԭ��Ʒ�������İٷ�����,��ش��������⣺

��5����������������̼Ԫ�صĻ��ϼ�Ϊ��
��6��A��B������Ӧ�Ļ�ѧ����ʽΪ ��
��7����ȷ�о�������B��Cʵ���Ƿ��������еģ�ÿһ��ֻ�ͷ�һ�����壬�ڶ����ͷŵ��������Է��������ϵ�һ���Ĵ����һ���ͷŵ����廯ѧʽΪ�� ���ͷŵڶ�������ʱ����Ӧ�Ļ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| ������ | SO42-��NO3-��Cl- |
| ������ | Fe3+��Fe2+��NH4+��Cu2+��Al3+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ģ���� ���ͣ�ʵ����
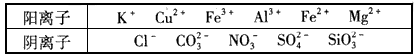
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com