
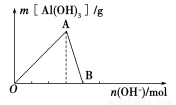
��ͼ��ʾΪһ����AlCl3��Һ�м���NaOH��Һ����Al(OH)3��ɫ������������NaOH�����ʵ���֮��Ĺ�ϵ���ߡ�
��ش��������⣺
��1��A��ʱ�Ѳμӷ�Ӧ��AlCl3��NaOH�����ʵ���֮��Ϊ________��
��2��AB����������ʾ�ķ�Ӧ�����ӷ���ʽΪ_________________________��
��3����B�����ɵ���Һ��ͨ�������̼���ɹ۲쵽��������_______________________��
��4������0.1 mol NH4Al(SO4)2����Һ����μ���5 mol��L��1 NaOH��Һ����ʼ������Һ�г��ְ�ɫ�����������ࣻһ��ʱ����д̼�����ζ�������ݳ�������ɫ�������ٲ�������ʧ��������ͼ�л������ɳ��������ʵ��������NaOH��Һ����Ĺ�ϵʾ��ͼ��

 ��ʦָ����ĩ��̾�ϵ�д�
��ʦָ����ĩ��̾�ϵ�д� �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ��ӱ�ʡ�߶�����ѧ���Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��2A+B 3C+4D��Ӧ�У���ʾ�÷�Ӧ���������ǣ���
3C+4D��Ӧ�У���ʾ�÷�Ӧ���������ǣ���
A��v(A)=0.5 mol/(L��min) B��v(B��=0.3 mol/(L��min)
C��v(C��=0.8 mol/(L��min) D��v(D��=1 mol/(L��min)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��㶫ʡ�����и���8���¿����ۻ�ѧ�Ծ��������棩 ���ͣ������
ij��ɫ����Һ�п��ܴ�������Ag����Mg2����Cu2���еļ������ӡ�
��1�������κ�ʵ��Ϳ��Կ϶�ԭ��Һ�в����ڵ�������_________�������� ��
��2��ȡ����ԭ��Һ�������ϡ���ᣬ�а�ɫ�������ɣ��ټ������ϡ���ᣬ��ɫ��������ʧ��˵��ԭ��Һ�п϶��е�������________���йص����ӷ���ʽΪ_____________��
��3��ȡ��2������Һ�������NaOH��Һ�����ְ�ɫ������˵��ԭ��Һ�п϶����ڵ�������_________���йط�Ӧ�����ӷ���ʽΪ ��
��4��ԭ��Һ�п��ܴ������ڵ�������������A��D�еģ�����ţ�_________��
A��Cl�� B��NO3�� C��CO32�� D��OH��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡЭ���������ٵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
�ҹ���ǭ�����ؽ���������й��������ҵ��еĵ���,����Ϊ�й��ġ������ǡ��������̵��۵�ܸ�,�������ȷ��Ƶô���,���õ�ⷨ�Ƶô��Ľ����̡���������ұ��������,����Ϊ��
A���۵�� B�����л�ԭ��
C���������� D��ұ����Ӧ�зų���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ɽ��ʡЭ���������ٵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
��״�������ֵ����ʵ����Ŀ�ȼ���干1.68L������һ����������������������ȫȼ�ա���������ͨ����������ʯ��ˮ���õ��İ�ɫ��������Ϊ15.0g������������ʯ������ȼ�ղ������9.3g���������ֻ���������Ϊ�� ��
A��H2��C2H4 B��CO��C2H4 C��CO ��C3��6 D��H2��C4H6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ����8���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��������Fe3O4��ĩ���Ƴ����ȼ����ֳ����ȷݡ�
��һ��ֱ�ӷ����������ռ���Һ�У���ַ�Ӧ��ų������ڱ�״���µ����ΪV1��
��һ���ڸ�����ǡ�÷�Ӧ��ȫ����Ӧ��Ļ���������������ᷴӦ�ų��������ڱ�״���µ����ΪV2��
��һ��ֱ�ӷ��������������У���ַ�Ӧ��ų������ڱ�״���µ����ΪV3��
����˵����ȷ����( )
A��V1��V3>V2 B��V2>V1��V3 C��V1��V2>V3 D��V1>V3>V2
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ����8���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
(2016�������ģ)�������ʵ�����þ������ϣ�ȡ�������û�����ķݣ��ֱ�ӵ�������������Һ�У���ַ�Ӧ�ų�����������( )
A��3 mol��L��1 HCl B��4 mol��L��1 HNO3 C��8 mol��L��1 NaOH D��18 mol��L��1 H2SO4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017��ӱ�ʡ�����и�����ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ��ƶ���
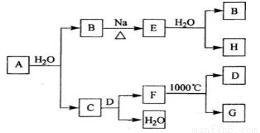
A����Ȼ�������㷺�Ģ�A��Ԫ�أ����Ի�����F���ڡ��ӵ���A��ʼ������һϵ�л�ѧ��Ӧ������ͼ��ʾ��
��ش��������⣺
��1��A��ˮ��Ӧ�Ļ�ѧ����ʽΪ_____________��E��ˮ��Ӧ�Ļ�ѧ����ʽΪ___________��
��2��F�Ļ�ѧʽΪ___________��G��D�ĵ���ʽ�ֱ�Ϊ_____________��____________��
��3��D��H��Ӧ�������ɵ�����_____________ (�ѧʽ)��
��4��ʵ�������У�����FΪԭ���Ʊ�����A������һ���Ʊ�����_________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�긣��ʡ�����и�һ���£����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
���н�����������ϡ���ᣬ�������Ƴɵ������ʺ�����ʢװŨ������ǣ� ��
A��п B���� C��ͭ D����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com