
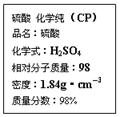
��ͼ�������Լ�ƿ��ǩ�ϵ����ݣ�
 �Ÿ���������ʵ���Ũ����
mol/L��
�Ÿ���������ʵ���Ũ����
mol/L��
��ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ����Ҫ
240 mL 4.6 mol/L��ϡH2SO4������Ҫȡ mL�ĸ����ᣬ
�������������ϡ�͵�ʵ�����Ϊ
��
��������4.6 mol/LϡH2SO4�Ĺ����У����������������Һ���ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��δ����ȴ������Һע������ƿ�У� ��
������ƿ��1mol/LϡH2SO4��ϴ�� ���۶���ʱ���ӹ۲�Һ�� ��
�ȳ����£�ijѧ��ȡ�������Լ�ƿ�е�������һ�ྻ�Թ��У����뼸Ƭ���������Ƭ����û�����ݲ�����Ҳδ������Ƭ�ܽ⣬������ͬѧ������ԭ�� ��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ͼ�������Լ�ƿ��ǩ�ϵ����ݡ�
(1)����������ʵ���Ũ��Ϊ ��
(2)ʵ�����ø���������240mL0.46mol/L��ϡ���ᣬ��
����Ҫ����������Ϊ mL��
�������������� A.�ձ� B.100mL��Ͳ C.250mL����ƿ D.500mL����ƿ E.������ F.������ƽ�������룩G.10mL��ͲH.��ͷ�ιܣ�����ʱ������ʹ�õ������� ������ţ���
�����ƹ������м����ؼ��IJ���Ͳ�������ͼ��ʾ��������ʵ�鲽��A��F��ʵ������Ⱥ�������� ��
�ܸ�ͬѧʵ�����Ƶõ���Ũ��Ϊ0.45mol/L�����ܵ�ԭ����
A����ȡŨH2SO4ʱ���ӿ̶� B������ƿϴ����δ�����ﴦ��
C��û�н�ϴ��Һת������ƿ D������ʱ���ӿ̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ�����У��һ5�½μ�⻯ѧ�Ծ����������� ���ͣ���ѡ��
��ͼ�������Լ�ƿ��ǩ�ϵIJ������ݡ��ݴ�����˵���У���ȷ���� �� ����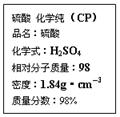
| A������200 mL 4.6 mol/L��ϡ������ȡ������50 mL |
| B��1 mol Zn�������ĸ����ᷴӦ����2 g H2 |
| C��������������������������� |
| D������С�Ľ������ὦ��Ƥ���ϣ�Ӧ������NaOH��Һ��ϴ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���㽭ʡ̨����ѧ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��10�֣���ͼ�������Լ�ƿ��ǩ�ϵ����ݡ�
(1)����������ʵ���Ũ��Ϊ ��
(2)ʵ�����ø���������240mL0.46mol/L��ϡ���ᣬ��
����Ҫ���������� Ϊ mL��
Ϊ mL��
�������������� A.�ձ�B.100mL��ͲC.250mL����ƿD.500mL����ƿE.������F.������ƽ�������룩G.10mL��ͲH.��ͷ�ιܣ�����ʱ������ʹ�õ������� ������ţ���
�����ƹ������м����ؼ��IJ���Ͳ�������ͼ��ʾ��������ʵ�鲽��A��F��ʵ������Ⱥ�������� ��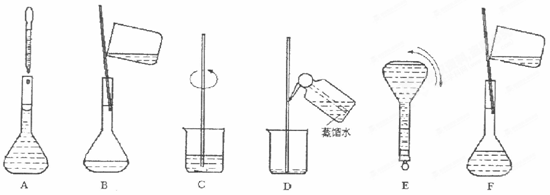
�ܸ�ͬѧʵ�����Ƶõ���Ũ��Ϊ0.45mol/L�����ܵ�ԭ����
A����ȡŨH2SO4ʱ���ӿ̶� B������ƿϴ����δ�����ﴦ��
C��û�н�ϴ��Һת������ƿ D������ʱ���ӿ̶�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015���Ϻ�����У��һ5�½μ�⻯ѧ�Ծ��������棩 ���ͣ�ѡ����
��ͼ�������Լ�ƿ��ǩ�ϵIJ������ݡ��ݴ�����˵���У���ȷ���� �� ����
A������200 mL 4.6 mol/L��ϡ������ȡ������50 mL
B��1 mol Zn�������ĸ����ᷴӦ����2 g H2
C���������������������������
D������С�Ľ������ὦ��Ƥ���ϣ�Ӧ������NaOH��Һ��ϴ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ������ѧ�ڵ�һ���¿���ѧ���� ���ͣ������
��7�֣���ͼ�������Լ�ƿ��ǩ�ϵ����ݣ�

��1������������ʵ���Ũ���� mol/L��
��2��ij��ѧ��ȤС������������ʵ�ʵ��̽��ʱ����Ҫ240 mL 4.6 mol/L��ϡH2SO4������Ҫȡ mL�ĸ����ᣬ�������������ϡ�͵�ʵ�����Ϊ ��
��3��������4.6 mol/LϡH2SO4�Ĺ����У����������������Һ���ʵ���Ũ���к�Ӱ�죨�ƫ�ߡ�����ƫ�͡�����Ӱ�족���� ��δ����ȴ������Һע������ƿ�У� ��
������ƿ��1mol/LϡH2SO4��ϴ�� ���۶���ʱ���ӹ۲�Һ�� ��
��4�������£�ijѧ��ȡ�������Լ�ƿ�е�������һ�ྻ�Թ��У����뼸Ƭ���������Ƭ����û�����ݲ�����Ҳδ������Ƭ�ܽ⣬������ͬѧ������ԭ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com