
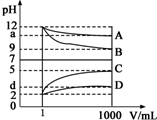
 ����A��DΪCH3COOH��NaOH��HCl��NH3•H2O�еĸ�һ�֣������½������ʵ���Һ��1mLϡ�͵�1000mL��pH�ı仯��ϵ��ͼ��ʾ����ش��������⣺
����A��DΪCH3COOH��NaOH��HCl��NH3•H2O�еĸ�һ�֣������½������ʵ���Һ��1mLϡ�͵�1000mL��pH�ı仯��ϵ��ͼ��ʾ����ش��������⣺���� ����ͼ�����߱仯��֪����Һ��1mLϡ�͵�1000mL��B��pH��С��3��C��pH������3��AD��pH�仯С��3��˵��AΪһˮ�ϰ���BΪNaOH��CΪHCl��DΪ���ᣬ
��1���������Ϸ������н��
��2����ˮ�����ᷴӦ�����Ȼ�狀�ˮ���ݴ�д����Ӧ�õ����ӷ���ʽ��
��3������������������Һ��Ӧ���ɴ����ƣ���������Ӳ���ˮ�⣬��Һ��ʾ���ԣ���ϵ���غ��жϸ�����Ũ�ȴ�С��
��4��a������CH3COOH��Һ�еĵ���غ��жϣ�
b��0.1mol/L ��CH3COOH ��Һ��ˮϡ�ͣ�������Ũ�ȼ�С�����ˮ�����ӻ������������������Ũ�ȵı仯��
c����������CH3COONa���壬��Һ�д�������ӵ�Ũ��������ƽ�������ƶ���
d�������£�pH=2��CH3COOH��Һ��pH=12��NaOH��Һ�������ϣ�����Ϊ���ᣬ����Һ�д����������Һ��ʾ���ԣ�
��� �⣺��1������ͼ�����߱仯��֪����Һ��1mLϡ�͵�1000mL��B��pH��С��3��C��pH������3��AD��pH�仯С��3��˵��AΪһˮ�ϰ���BΪNaOH��CΪHCl��DΪ���ᣬ
�ʴ�Ϊ��NaOH��HCl��
��2��һˮ�ϰ������ᷴӦ�����Ȼ�狀�ˮ����Ӧ�����ӷ���ʽΪ��NH3•H2O+H+=NH4++H2O��
�ʴ�Ϊ��NH3•H2O+H+=NH4++H2O��
��3�������ʵ�����NaOH��CH3COOH��ֻ�Ϻ�����CH3COONa��Һ����������Ӳ���ˮ�⣬��Һ�ʼ��ԣ���c��OH-����c��H+�������ݵ���غ��֪c��Na+����c��CH3COO-������Һ������Ũ�ȴ�СΪ��c��Na+����c��CH3COO-����c��OH-����c��H+����
�ʴ�Ϊ��c��Na+����c��CH3COO-����c��OH-����c��H+����
��4��a��CH3COOH��Һ�У����ݵ���غ��֪��c��H+��=c��OH-��+c��CH3COO?������a��ȷ��
b��0.1mol/L ��CH3COOH��Һ��ˮϡ�ͣ���Һ��������Ũ�ȼ�С������ˮ�����ӻ����䣬����Һ��c��OH-������b����
c��CH3COOH��Һ�м�������CH3COONa���壬���������Ũ��������ƽ�������ƶ�����c����
d�������£�pH=2��CH3COOH��Һ��pH=12��NaOH ��Һ�������Ϻ������������Һ�����ԣ���Һ��pH��7����d��ȷ��
�ʴ�Ϊ��b��
���� ���⿼������Һ���������ҺpH�Ĺ�ϵ������Ũ�ȴ�С�Ƚϣ���Ŀ�Ѷ��еȣ���ȷ��Һ���������ҺpH�Ĺ�ϵΪ���ؼ���ע������Ӱ��������ʵĵ���ƽ������أ��ܹ����ݵ���غ㡢�����غ��֪ʶ�ж�����Ũ�ȴ�С��


 ��ĩ�����ϵ�д�
��ĩ�����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
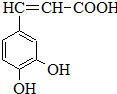 �����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã�
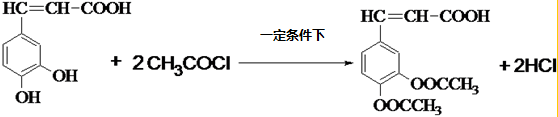
�����ᣨ��ͼ����������������ҩ�У���Ұ���ܲ�����Ҷˮ�ա�����ľ���ĵȣ���������ֹѪ���ã��ر�������ֹѪЧ���Ϻã�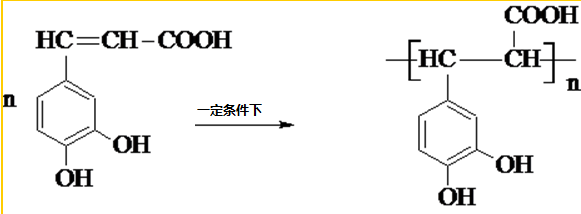 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

| �������� | Fe3+ | Cu2+ |
| �������↑ʼ����ʱ��pH | 1.9 | 4.7 |
| ����������ȫ����ʱ��pH | 3.2 | 6.7 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
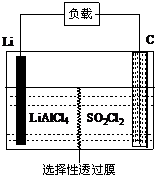 Ϊ�˹����ִ�����ij���¿������з���һ�����͵�Li-SO2Cl2���õ�أ�����ɽṹʾ��ͼ��ͼ��ʾ����֪����ܷ�ӦΪ��2Li+SO2Cl2�T2LiCl+SO2��������˵���в���ȷ���ǣ�������
Ϊ�˹����ִ�����ij���¿������з���һ�����͵�Li-SO2Cl2���õ�أ�����ɽṹʾ��ͼ��ͼ��ʾ����֪����ܷ�ӦΪ��2Li+SO2Cl2�T2LiCl+SO2��������˵���в���ȷ���ǣ�������| A�� | ��صĸ�����ӦΪ��2Li-2e-�T2Li+ | |
| B�� | ����ʱ��������﮵缫�����ߡ����ء�̼�� | |
| C�� | �����缫��ӦΪ��SO2Cl2+2e-�T2Cl-+SO2�� | |
| D�� | ��ع���ʱ������̼�����濴�����ݲ��� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
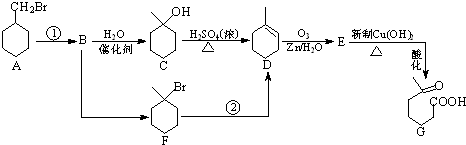
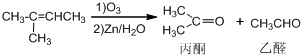
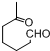 +NaOH+2Cu��OH��2$\stackrel{��}{��}$
+NaOH+2Cu��OH��2$\stackrel{��}{��}$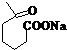 +Cu2O��+3H2O��
+Cu2O��+3H2O�� ����D��ૣ�
����D��ૣ�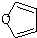 ��Ҳ���Է�����Diels-Alder��Ӧ�����û�ѧ��Ӧ����ʽΪ
��Ҳ���Է�����Diels-Alder��Ӧ�����û�ѧ��Ӧ����ʽΪ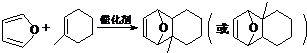 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | NaHCO3��Һ�ʼ��Ե�ԭ��HCO3-+H2O?CO32-+H3O+ | |
| B�� | ��������Һ����μ���Ba��OH��2��Һ�������������Al3++2SO42-+2Ba2++4OH-�T2BaSO4��+AlO2-+2H2O | |
| C�� | �����������ڴ�����Һ��Al��OH��3+3H+�TAl3++3H2O | |
| D�� | �Ȼ�����Һ�м��������ˮAl3++4OH-�TAlO2-+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������pH=2��������pH=12�İ�ˮ�������϶��� | |
| B�� | ����Һ�����ɵ����ʵ���Ũ�ȵ�����Ͱ�ˮ�������϶��� | |
| C�� | ����������ˮ����Һ������Ũ�ȿ���Ϊ��c ��NH4+����c ��Cl-����c ��OH-����c��H+�� | |
| D�� | ����Һ��c ��NH4+��=c ��Cl-��+c ��OH-��-c��H+�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ����һ�����ʵ���Ũ�ȵ���Һ��������ƿ��ˮ��Һ����̶���1��2 cmʱ�����ý�ͷ�ιܶ��� | |
| B�� | ��ij��Һ���ȼ���Ba��NO3��2��Һ���ټ���������HNO3��Һ��������ɫ�����������Һ��һ������SO42- | |
| C�� | ��Һ����ʱ�����²�Һ���ȷų���Ȼ��ر����������ϲ�Һ����Ͽڵ��� | |
| D�� | ����֬������������Һ��ϳ�ַ�Ӧ���ټ����ȵı���ʳ��ˮ�����������������Ҫ�ɷ� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com