
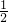 ��1.0mol/L=1.0 mol/L��c��H2SO4��=
��1.0mol/L=1.0 mol/L��c��H2SO4��=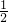 ��1.0mol/L=0.5mol/L��
��1.0mol/L=0.5mol/L��


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��㶫�������и�һ��ѧ����ĩ���Ի�ѧ�Ծ����������� ���ͣ�������
��2.0mol/L CuSO4��Һ��1.0mol/L H2SO4��Һ�������ϣ������Ϻ���Һ��������ڻ��ǰ������Һ�����֮�ͣ������㣺
��1�������Һ��H+��Cu2+��SO42�������ʵ���Ũ�ȣ�
��2������Һ�м����������ۣ����㹻����ʱ���������ʣ�࣬���ˣ���ʱ��Һ�е�Fe2+�����ʵ���Ũ�ȣ�
��3��ȡ100mL����Һ�������м����������ᣬ�ټ�����������������Һ���к��ɫ�������ɡ����ˣ����ȳ��������������ٱ仯���õ�����ɫ�IJ�����д�������Ͳ����Ļ�ѧʽ����������������������Ʋ�����������ʧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015��㶫�������и�һ��ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�������
��2.0mol/L CuSO4��Һ��1.0mol/L H2SO4��Һ�������ϣ������Ϻ���Һ��������ڻ��ǰ������Һ�����֮�ͣ������㣺
��1�������Һ��H+��Cu2+��SO42�������ʵ���Ũ�ȣ�
��2������Һ�м����������ۣ����㹻����ʱ���������ʣ�࣬���ˣ���ʱ��Һ�е�Fe2+�����ʵ���Ũ�ȣ�
��3��ȡ100mL����Һ�������м����������ᣬ�ټ�����������������Һ���к��ɫ�������ɡ����ˣ����ȳ��������������ٱ仯���õ�����ɫ�IJ�����д�������Ͳ����Ļ�ѧʽ����������������������Ʋ�����������ʧ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008-2009ѧ�걱��101�и�һ���ϣ���ĩ��ѧ�Ծ��������棩 ���ͣ������
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com