



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
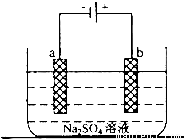
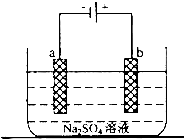 ��1������������ɴ�ʹ�õ��Ǽ�������ȼ�ϵ�أ������������������������������������������NaOH��Һ����д���÷�Ӧ�����ĵ缫��Ӧʽ��
��1������������ɴ�ʹ�õ��Ǽ�������ȼ�ϵ�أ������������������������������������������NaOH��Һ����д���÷�Ӧ�����ĵ缫��Ӧʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
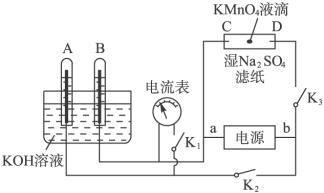
��1����______�������������������Ӧʽ�� ����______�������������������Ӧʽ��_________________���۵�ط�Ӧʽ_________________________________��
��2����ͼ��ʾװ�ã�A��B�еĵ缫Ϊ��Ķ��Ե缫��C��DΪ����ʪ��Na2SO4��ֽ���ϵIJ��У���Դ��a��b��������A��B�г���KOH��Һ������KOH��Һ��ˮ���У��ж�K1���պ�K2��K3ֱͨ���磬�ش��������⣺
���жϵ�Դ������������aΪ___________����bΪ��___________��
����ʪ��Na2SO4��Һ��ֽ�����ĵ�KMnO4Һ�Σ���ʲô����______________________��
��д���缫��Ӧʽ��A��______________________��C��______________________��
����A���������������ڱ�״����Ϊx L����C���ϲ����������ԼΪ___________g��
�������һ��ʱ���A��B�о��������Χ�缫����ʱ�ж�K2��K3���պ�K1�����������ָ���Ƿ��ƶ�___________����ǡ�������������ָ�벻�ƶ�˵�����ɣ���ָ���ƶ�Ҳ˵�����ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��1������������ɴ�ʹ�õ�����KOHΪ���ʵ��⡢��ȼ�ϵ�أ�����ͼ����������Ҫ����գ�
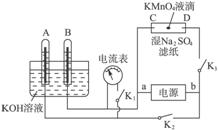
��1����__________�������������������Ӧʽ������__________�������������������Ӧʽ��_________________���۵�ط�Ӧʽ_________________________________��
��2����ͼ��ʾװ�ã�A��B�еĵ缫Ϊ��Ķ��Ե缫��C��DΪ����ʪ��Na2SO4��ֽ���ϵIJ��У���Դ��a��b��������A��B�г���KOH��Һ������KOH��Һ��ˮ���У��ж�K1���պ�K2��K3ֱͨ���磬�ش��������⣺
���жϵ�Դ������������aΪ___________����bΪ��___________��
����ʪ��Na2SO4��Һ��ֽ�����ĵ�KMnO4Һ�Σ���ʲô����______________________��
��д���缫��Ӧʽ��A��______________________��C��______________________��
����A���������������ڱ�״����Ϊx L����C���ϲ����������ԼΪ___________g��
�������һ��ʱ���A��B�о��������Χ�缫����ʱ�ж�K2��K3���պ�K1�����������ָ���Ƿ��ƶ�___________����ǡ�������������ָ�벻�ƶ�˵�����ɣ���ָ���ƶ�Ҳ˵�����ɡ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
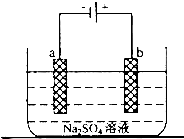
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com