
���������ؾ���Kx[Fe��C2O4��y]��3H2O��������Ӱ����ɫӡˢ��ʵ�����Ʊ����������ؾ����ʵ���������£�

��1����������ϡ�����Ʊ�FeSO4��7H2O��������______������������___________________________________________________��
��2�����������У���������һ������FeC2O4��2H2O��������ˮϴ�ӡ���������Ƿ�ϴ�Ӹɾ��ķ�����_________________________________________________________________��
��3���ⶨ���������ز�Ʒ��Fe3��������C2O42-������ʵ�鲽�����£�

����1��ȷ��ȡ���Ʋ��������ؾ���a g��Լ1.5 g�������250 mL����Һ��
����2������Һ����ȡ25.00 mL����Һ����ƿ�У�����6 mol��L��1 HCl 10 mL��������70��80 ����������SnCl2TiCl3���ϻ�ԭ����Fe3��ȫ����ԭΪFe2��������MnSO4��Һ10 mL����75��80 ������0.010 00 mol��L��1 KMnO4����Һ�ζ����յ㣨Cl�������뷴Ӧ������C2O42-ȫ��������CO2��Fe2��ȫ��������Fe3����¼�����
����3������
����4���ظ���������2������3���Ρ�
������2����ʱ������Ҫʹ����ͼ��ʾ�����е�________������ţ���
������2��MnSO4��Һ��������________���ζ��յ��������______________________________________��
���ڲ������Լ������ǰ���£�����3��Ŀ����_________________________��
��1��������ֹFe2������������
��2��ȡ�������һ��ϴ��Һ������BaCl2��Һ�������ְ�ɫ������˵������û��ϴ�Ӹɾ�����֮��������ϴ�Ӹɾ�������������Ҳ�ɣ�
��3����ad��������������Һ��Ϊdz��ɫ���Ұ���Ӳ���ɫ�����ⶨC2O42-�ĺ���
����������1������ϡ�����Ʊ�FeSO4��7H2O������һ�����ʹ�������Ҫ�����ᣬ��Ϊ�ڻ�þ���Ĺ���������Fe2����ˮ�⡣��2������ϴ���Ƿ�ɾ�����Ҫ�dz����������Ŀ����������Ƿ���ڣ����ȷ������ʿ�����K2SO4������ͨ������SO42-����ϴ���Ƿ�ɾ�����3����ע��ⶨ������Ҫ�����¶ȣ�ѡ���¶ȼƣ�������ͨ������KMnO4�ζ���ѡ����ʽ�ζ��ܡ����ζ�������MnO4-����ԭΪMn2�����ȼ���Mn2�����������������ζ��յ�ʱ��KMnO4��������ʱ��Һ��dz��ɫ���Ұ�����ڲ���ɫ������ʵ���Ŀ���DzⶨFe3����C2O42-�����ⶨ�����У����Ƚ�Fe3����ԭΪFe2����Ȼ������KMnO4�ζ�����Fe2����C2O42-�����ֱ��Fe2����C2O42-���Ե�����ֻ����������˻���Ҫ�ζ���Fe2����C2O42-�������֪�ζ�C2O42-�����ף���Ϊ������ȡ��Һ��ֱ�ӵζ�����Ϊ��ʱ��ΪFe3������KMnO4��Ӧ��


 �����ܿ����ϵ�д�
�����ܿ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2014ѧ���һ��ѧ�˽̰����2 1.2.1���ӵ��Ų�Ԫ����������ϰ���������棩 ���ͣ�ѡ����
��a��b��c��d����Ԫ�أ�a��b�������Ӻ�c��d�������ӵ��Ӳ�ṹ��ͬ����֪b�������Ӱ뾶����a�������Ӱ뾶��c�������ӱ�d�������ӻ�ԭ����ǿ��������Ԫ�ص�ԭ��������С��ϵ�� (����)��
A��a>b>d>c B��b>a>c>d C��b>a>d>c D��c>b>a>d
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014ѧ���һ��ѧ�˽̰����2 1.1.1Ԫ�����ڱ���ϰ���������棩 ���ͣ������
�±���Ԫ�����ڱ���һ���֡��������е���ĸ�ֱ����ijһ��ѧԪ�ء�

���ϱ��е���ĸ���Żش��������⣺
(1)����±��Ԫ�ص���_________________________________________��
(2)����ϡ������Ԫ�ص���_____________________________________��
(3)���ڵ���A����Ԫ�ص���___________________________________��
(4)д��cԪ�������ڱ��е�λ��_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ�������ʡ������ѧ����ĩ�������ۻ�ѧ�Ծ��������棩 ���ͣ�ѡ����
X��Y��Z��W��Ϊ������Ԫ�أ����������ڱ��е�λ����ͼ��ʾ����Yԭ�ӵ��������������ڲ��������3��������˵������ȷ����

A��ԭ�Ӱ뾶��W>Z>Y>X
B��������ʵ���Ũ�ȵ���̬�⻯����Һ��pH: X>Z>W
C������������Ӧˮ��������ԣ�Z>W>X
D������Ԫ�صĵ����У�Z���ʵ��۷е����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ�������ѵ�� ר��1����������ʷ��༰��ѧ������ϰ���������棩 ���ͣ�ѡ����
����˵������ȷ��������������
A������[KAl��SO4��2��12H2O]��ˮ�����γ�Al��OH��3���壬��������ˮ��
B�������뺣���������γ�ͨ���뽺��������й�
C��������Һ���ж����ЧӦ
D������е�1 mol��L��1 NaOH��Һ�еμ�FeCl3������Һ�Ʊ�Fe��OH��3����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� �߿�ģ������2��ϰ���������棩 ���ͣ�ѡ����
�����£���20 mL 0.1 mol��L��1CH3COOH��Һ����μ���0.1 mol��L��1NaOH��Һ����pH�仯������ͼ��ʾ�������¶ȱ仯��������˵���д�����ǣ���������

A��a��b֮�����Һ��ֻ���ڣ�c��CH3COO������c��Na������c��H������c��OH����
B��b��c֮�����Һ�в����ڣ�c��CH3COO������c��H������c��Na������c��OH����
C��b��ʱ��V��NaOH��Һ����20 mL����c��CH3COO������c��Na����
D��a��b��c���ʾ����Һ�� �����
�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� �߿�ģ������2��ϰ���������棩 ���ͣ�ѡ����
��NAΪ�����ӵ�������ֵ������������ȷ���ǣ���������

P4���ӽṹģ��
A�����³�ѹ�£�16 g14CH4��������������Ϊ8NA
B����״���£�22.4 L CCl4�������ķ�����ΪNA
C����1 L 1.0 mol��L��1 NaCl��Һ�к���NA��NaCl����
D��6.2 g���ף�����ʽΪP4�����ӽṹ��ͼ��ʾ����������P��P����ĿΪ0.3NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� �߿�ģ������1��ϰ���������棩 ���ͣ�ѡ����
��һ��ɺ���з�����Ļ��Ժ��Ȼ�����punaglandin���к�ǿ�Ŀ������ԣ����Ľṹ��ʽ��ͼ���йظû������˵����ȷ���ǣ���������

A�����Ӻ���4������̼ԭ��
B����ʹ���Ը��������Һ��ɫ
C��������3�ֺ���������
D������������̼ԭ�ӿ��Դ���ͬһƽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013-2014ѧ��߿���ѧ���ָ�ϰ����ר�� ��7��ˮ��Һ�е�����ƽ����ϰ���������棩 ���ͣ�ѡ����
25 ��ʱ����Ũ��Ϊ0.100 0 mol��L��1��NaOH��Һ�ζ�20.00 mLŨ�Ⱦ�Ϊ0.100 0 mol��L��1��������HX��HY��HZ���ζ�������ͼ��ʾ������˵����ȷ����(����)��

A������ͬ�¶��£�ͬŨ�ȵ���������Һ�ĵ�������˳��HZ��HY��HX
B�����ݵζ����ߣ��ɵ�Ka(HY)��10��5
C��������HX��HY��Һ�������Ϻ���NaOH��Һ�ζ���HXǡ����ȫ��Ӧʱ��c(X��)��c(Y��)��c(OH��)��c(H��)
D��HY��HZ��ϣ��ﵽƽ��ʱc(H��)��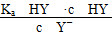 ��c(Z��)��c(OH��)
��c(Z��)��c(OH��)
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com