

| ||
| ||
| ||


 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ˮ��һ�ַḻ����Դ����ҵ�ϴӺ�ˮ�п���ȡ���������ʣ��㷺Ӧ��������������Ƽ��ȷ��档��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��
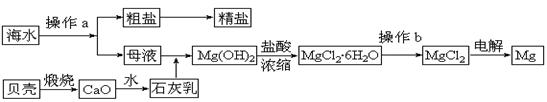
�ش��������⣺
(1)����ͼ�в���a������Ϊ ��
(2)��ҵ�ϴӺ�ˮ����ȡ��NaCl����������ȡ���꣬���Ҫ�������£���ʳ��ˮ����ͨ������A����ͨ������B����ַ�Ӧ����˵õ�����C����ҺD��������C���ռ����Ƶô��
������A��B��CO2��NH3��������AӦ�� ���ѧʽ����
����ҺD����Ҫ����NH4Cl��NaHCO3�����ʣ���ҵ��������ҺD��ͨ��NH3��������ϸСʳ�ο�������ȴ����������NaHCO3�ĸ���ƷNH4Cl���壬��ͨ��NH3��������
��
(3)þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ��
����Ҫ��֤������ˮMgCl2�в���NaCl����IJ��������ǣ�
��
�ڲ���b���� ��Χ�н��У����ڿ����м��ȣ��������Mg(OH)Cl��д���йط�Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10�֣���ˮ��һ�ַḻ����Դ����ҵ�ϴӺ�ˮ�п���ȡ���������ʣ��㷺Ӧ��������������Ƽ��ȷ��档��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��
�ش��������⣺
��1������ͼ�в���a�������ᾧ����� ����������ƣ���
��2����ҵ�ϴӺ�ˮ����ȡ��NaCl����������ȡ���꣬���Ҫ�������£���ʳ��ˮ��
��ͨ������A����ͨ������B����ַ�Ӧ����˵õ�����C����ҺD��������C���ռ����Ƶô��
������A��B��CO2��NH3��������AӦ�� ���ѧʽ����
����ҺD����Ҫ����NH4Cl��NaHCO3�����ʣ���ҵ��������ҺD��ͨ��NH3��������ϸСʳ�ο�������ȴ����������NaHCO3�ĸ���ƷNH4Cl���壬��ͨ��NH3�������� ������������������ ��
��3��þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ��
����Ҫ��֤������ˮMgCl2�в���NaCl����IJ��������ǣ�
��
�ڲ���b���� ��Χ�н��У����ڿ����м��ȣ��������Mg(OH)Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��2011ѧ���㽭ʡ���������ѧУ�߶��꼶��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���ˮ��һ�ַḻ����Դ����ҵ�ϴӺ�ˮ�п���ȡ���������ʣ��㷺Ӧ��������������Ƽ��ȷ��档��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��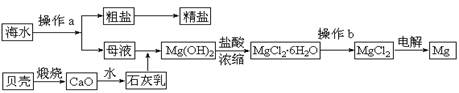
�ش��������⣺
��1������ͼ�в���a�������ᾧ����� ����������ƣ���
��2����ҵ�ϴӺ�ˮ����ȡ��NaCl����������ȡ���꣬���Ҫ�������£���ʳ��ˮ��
��ͨ������A����ͨ������B����ַ�Ӧ����˵õ�����C����ҺD��������C���ռ����Ƶô��
������A��B��CO2��NH3��������AӦ�� ���ѧʽ����
����ҺD����Ҫ����NH4Cl��NaHCO3�����ʣ���ҵ��������ҺD��ͨ��NH3��������ϸСʳ�ο�������ȴ����������NaHCO3�ĸ���ƷNH4Cl���壬��ͨ��NH3�������� ������������������ ��
��3��þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ��
����Ҫ��֤������ˮMgCl2�в���NaCl����IJ��������ǣ�
��
�ڲ���b���� ��Χ�н��У����ڿ����м��ȣ��������Mg(OH)Cl��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012���㽭ʡ���������ѧУ�߶��꼶��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10�֣���ˮ��һ�ַḻ����Դ����ҵ�ϴӺ�ˮ�п���ȡ���������ʣ��㷺Ӧ��������������Ƽ��ȷ��档��ͼ��ij�����Ժ�ˮ��Դ�����ۺ����õ�ʾ��ͼ��
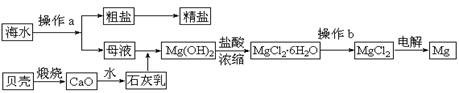
�ش��������⣺
��1������ͼ�в���a�������ᾧ����� ����������ƣ���
��2����ҵ�ϴӺ�ˮ����ȡ��NaCl����������ȡ���꣬���Ҫ�������£���ʳ��ˮ��
��ͨ������A����ͨ������B����ַ�Ӧ����˵õ�����C����ҺD��������C���ռ����Ƶô��
������A��B��CO2��NH3��������AӦ�� ���ѧʽ����
����ҺD����Ҫ����NH4Cl��NaHCO3�����ʣ���ҵ��������ҺD��ͨ��NH3��������ϸСʳ�ο�������ȴ����������NaHCO3�ĸ���ƷNH4Cl���壬��ͨ��NH3�������� ������������������ ��
��3��þ��һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�Ӻ�ˮ����ȡ��
����Ҫ��֤������ˮMgCl2�в���NaCl����IJ��������ǣ�
��
�ڲ���b���� ��Χ�н��У����ڿ����м��ȣ��������Mg(OH)Cl��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com