
���к͵ζ���ȷ��ij�ռ���Ʒ��Ũ�ȣ��Ը���ʵ��ش��������⣺
��1��ȷ��ȡһ��������Ʒ�����500 mL������Һ������ʱ����Ʒ�ɷ�����������������ĸ���ϳ���
��A��С�ձ� ��B���ྻֽƬ ��C��ֱ�ӷ���������
��2��ȷ��ȡ10.00ml����Һ����ƿ�У���0.2000mol��L-1���������ζ�������Һ������ѡ�� ��������ĸ����ָʾ��������A������ ��B��ʯ�� ��C����̪
��3����ѡ�ü�����ָʾ�����ζ��յ���ж�������
��ʱ��Һ�� �ԡ�
��4��0.2000mol��L-1������Ӧװ����ͼ��ʾ�� ����ס��ң��У�ͼ����ʾΪ�ζ�ǰҺ�棬ͼ����ʾΪ�ζ�����ʱҺ�棬����ͼʾ���㱻���ռ���Һ�����ʵ���Ũ���� mol��L-1��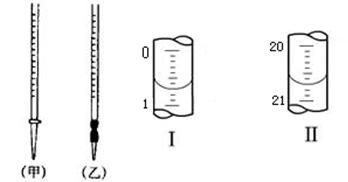
��5������ʵ�������Եζ���������ĺ�������ƫ�ߡ�����ƫ�͡�����Ӱ�족��
�ٹ۲���ʽ�ζ���Һ��ʱ����ʼ���ӣ��ζ��յ�ƽ�ӣ���ζ���� ��
��������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ����ζ���� ��
��1��(A) ��2��(B)
��3�����������һ�α�����Һʱ����Һ�պ��ɻ�ɫ��Ϊ��ɫ���Ұ�����ޱ仯 ��
��4���� 0.4000 ��5����ƫ�� ��ƫ��
���������������1�������������׳�������ʣ����Գ���ʱ����Ҫ���ձ��������
��2��ʯ��ı�ɫ��ΧΪ5��8����ɫ��Χ�ϴ�������ָʾ����
��3����μ��ü�����ָʾ�������ȵ���ɫ�ɻƱ�Ϊ��ɫ�����Եζ��յ���ж������ǵ��������һ�α�����Һʱ����Һ�պ��ɻ�ɫ��Ϊ��ɫ���Ұ�����ޱ仯�����ȵı�ɫ��Χ��3.1-4.4����ʱ��Һ�����ԡ�
��4������Ӧ������ʽ�ζ���ʢװ�����ѡ��ס���ͼ���Կ�������������Ϊ20.00mL������c(H+)V(H+)= c(OH-)V(OH-)���������NaOH��Ũ��Ϊ0.4000��
��5��c(OH-)=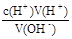 ����ʼ���ӣ��ζ��յ�ƽ�ӣ�����HCl�����������ֵ������ƫ�ߡ�������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ������HCl�����������ֵ������ƫ�ߡ�
����ʼ���ӣ��ζ��յ�ƽ�ӣ�����HCl�����������ֵ������ƫ�ߡ�������ƿ�ô���Һ��ϴ��Ȼ���ټ���10.00mL����Һ������HCl�����������ֵ������ƫ�ߡ�
���㣺����к͵ζ�
����������к͵ζ��ǻ�ѧʵ��Ļ����⣬����Ҫ���ӵζ��յ���жϡ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��0115 �¿��� ���ͣ������
| �������й�ʵ�����������ȷ����____������ţ��� �����������Һ�ζ����������������Һʱ��ˮϴ�����ʽ�ζ���δ������Һ��ϴ����ⶨ���ƫ�͡� �� ����һ�����ʵ���Ũ�ȵ���Һʱ����������ƿ�Ŀ̶��ߣ���ʹ���Ƶ�Ũ��ƫ�ߣ� �� ��Fe2(SO4)3��Һ�����������ɲ����գ����õ�����ɫ��ĩ �� ����ع�������Һ��ȴδ���ֽᾧʱ�������ò������ĥ������ڴ�ʹ���������� �ݿ���25ml��ʽ�ζ�����ȡ20.00mlKMnO4��Һ �ֱ��������pH����ͬ������ʹ����еμӵ�Ũ�ȵ�����������Һ����ȫ�к�ʱ���ĵ�����������Һ�����һ���� ��������������������ͭ���Ǻ�ɫ��ĩ�����������ϡ�ijУһ��ѧʵ��С��ͨ ��ʵ����̽����ɫ��ĩ��Fe2O3��Cu2O�������̽���������£� �������ϣ�Cu2O��һ�ּ������������ϡ��������Cu��CuSO4���ڿ����м�������CuO�� ������� ����1����ɫ��ĩ��Fe2O3 ����2����ɫ��ĩ��Cu2O ����3����ɫ��ĩ��Fe2O3��Cu2O�Ļ���� ���̽��ʵ�� ȡ������ĩ��������ϡ�����У���������Һ���ٵμ�KSCN�Լ��� ��1��������1��������ʵ��������_________________�� ��2�����μ�KSCN�Լ�����Һ�����ɫ����֤��ԭ�����ĩ��һ����������������������Ϊ����˵��������?_________________�������������(����д����Ӧ�ķ���ʽ)___________________�� ��3���������ĩ��ȫ�ܽ�������ڣ��μ�KSCN�Լ�ʱ��Һ�����ɫ����֤��ԭ�����ĩ��____________��д��������Ӧ�����ӷ���ʽ________________�� ̽������ ��ʵ�������ȷ����ɫ��ĩΪFe2O3��Cu2O�Ļ��� ��4��ʵ��С�����ü��ȷ��ⶨCu2O������������ȡag�����ĩ�ڿ����г�ּ��ȣ����������ٱ仯ʱ����������Ϊbg(b>a)����������Cu2O����������Ϊ________�� ��5��ʵ��С�������øú�ɫ��ĩ��ȡ�ϴ����ĵ���(CuSO4��5H2O)�����������ϵ�֪������Һ��ͨ��������Һ������Զ�ʹCu2+��Fe2+��Fe3+�ֱ����ɳ�����pH���£� | ||||||||||||
| ||||||||||||
| ʵ�����������Լ��ɹ�ѡ�� A����ˮ B��H2O2 C��NaOH D��Cu2(OH)2CO3 ʵ��С��������·��� | ||||||||||||
 | ||||||||||||
| �Իش� ���Լ�1Ϊ_______���Լ�2Ϊ________(����ĸ)�� �ڹ���X�Ļ�ѧʽΪ____________�� �۲���IΪ___________�� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com