
��15�֣�ij�����A������KAl��SO4��2��Al2O3��Fe2O3����һ�������¿�ʵ����ͼ��ʾ������֮��ı仯��
�ݴ˻ش��������⣺
��1��I��II��III��IV�IJ��ж�����Һ�ͳ����ķ����ȡ�ķ����� ��
��2������������ͼ��Ӧ��ϵ��д������B��C��D��E�������ʵĻ�ѧʽ
��������B ��C ��
����D ����ҺE ��
��3��д���١��ڡ��ۡ����ĸ���Ӧ����ʽ�������ӷ�Ӧ��д�����ӷ���ʽ��
�� ���� ��
�� �� �� ��
��1������ ��2��Al2O3��Al2O3��Fe2O3��Fe2O3��K2SO4��(NH4)2SO4
��3����Al2O3+2OH?=2AlO2-+H2O ��Al3++3NH3?H2O=Al(OH)3��+3NH4+
��AlO2-��H+��H2O��Al(OH) 3����Cl? ��2Al(OH) 3 Al2O3��3H2O
Al2O3��3H2O
�������������KAl��SO4��2������ˮ��Al2O3��Fe2O3��������ˮ�������A��ˮ�ܽ����Һ����KAl��SO4��2������CΪAl2O3��Fe2O3����ת����ϵͼ��֪�������C�м�NaOH��Һ��Fe2O3����Ӧ������DΪFe2O3��Al2O3����NaOH��Һ��Ӧ����NaAlO2����NaAlO2��Һ��ͨ��CO2�ɵ�Al��OH��3������Al��OH��3���ȷֽ����ɹ���BΪAl2O3������Һ�мӹ�����ˮ����Һ�������ˮ��Ӧ��Al3+���������õ�����������������Һ��EΪK2SO4����NH4��2SO4�������������ᾧ���õ�K2SO4�ͣ�NH4��2SO4��
��1����Һ�ͳ����ķ��뷽��Ϊ���ˡ�
��2������������֪BΪAl2O3��CΪAl2O3��Fe2O3��DΪFe2O3����ҺEΪK2SO4�ͣ�NH4��2SO4��
��3����Ӧ��Ϊ���ӷ�Ӧ��Al2O3+2OH?=2AlO2-+H2O����Ӧ��Ϊ���ӷ�Ӧ��Al3++3NH3?H2O=Al(OH)3��+3NH4+����Ӧ��Ϊ���ӷ�Ӧ��AlO2-��H+��H2O��Al(OH) 3����Cl?����Ӧ��Ϊ2Al��OH��3 Al2O3+3H2O��
Al2O3+3H2O��
���㣺���⿼�����ƶϡ����ʵķ��롢����ʽ����д��


 ������������ϵ�д�
������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
A��E����ѧ������5�ֻ����A��B�����������֮���ת����ϵ����ͼ��ʾ��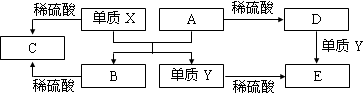
������˵����ȷ����
A��X��A��Ӧ�Ļ�ѧ����ʽ�ǣ�Al2O3 + 2Fe  Fe2O3 + 2Al
Fe2O3 + 2Al
B������D��Һ�еĽ��������ӵķ�Ӧ��Fe3++3SCN��= Fe(SCN)3��
C������Y��һ������������ˮ�����û���Ӧ
D�����ڻ�����B��C���������ᷴӦ��������Ӧ�����Ծ������Ի�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪A ��B��C��D֮���ת����ϵ��ͼ��ʾ������˵����ȷ���� ( )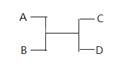
A����AΪFe��DΪ��������Bһ��Ϊ��
B����A��DΪ�����BΪˮ����Cһ�������嵥��
C����A��B��C��D��Ϊ������÷�Ӧһ�����ڸ��ֽⷴӦ
D����A��B��C��D��Ϊ10����������C�ǿ�ʹʪ��ĺ�ɫʯ����ֽ���������壬��D������һ����Һ̬
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��֪��AΪ�����к������ĵ��ʡ�������ͼת����ϵ���ش��������⣺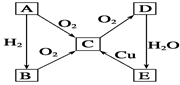
��1��д���������ʵĻ�ѧʽ��
A ��D ��
��2��E��C�ķ�Ӧ����ʽΪ ��
��3�����õ�E��Ũ��Һ�����ʻ�ɫ��ԭ���� ��
��4��ʵ������ȡB�Ļ�ѧ����ʽΪ____________________________________��
��5������1��00mol��L-1��E��Һ,����ȡ��10��00 mL����10��00 mL��Һ�����ʵ���Ũ��Ϊ mol��L-1����ǰ��ȡ����E��Һ���Ƴ�0��100mol��L-1��ϡ��Һ,����IJ����������ձ����������⣬���� �� �������ƹ����ж���ʱ���ӣ���������ҺŨ�� ���ƫ��ƫС������Ӱ�족����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����H2SO4��Ba(OH)2��Na2CO3����ˮ������Һ������ͼ��ʾ�����ϵ��ͼ��ÿ���߶����˵����ʿ��Է�����ѧ��Ӧ�������ƶϺ�������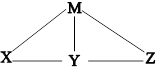
| A��Mһ����Ba(OH)2 | B��Y��������ˮ |
| C��Xһ����Na2CO3��Һ | D��Z������H2SO4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����10�֣����¿�ͼ��A��M����ѧ��ѧ���������ʣ�����A��E�ǽ�����F��JΪ���嵥�ʣ������Ϊ�����������Һ��CΪ����ɫ���壬DΪ����ɫ��ĩ��MΪ���ɫ���塣 
��1��B�Ļ�ѧʽ______��
��2��19��5gC��������ˮ��Ӧת�Ƶ��ӵ����ʵ���Ϊ______mol��
��3��д������C�ĵ���ʽ ��
��4��д���ڡ��۷�Ӧ�����ӷ���ʽ�� ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��G�����ʼ�Ĺ�ϵ��ͼ������B��DΪ��̬���ʡ�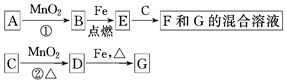
��ش��������⡣
(1)����C��E�����Ʒֱ�Ϊ �� ��
(2)��ѡ�ò�ͬ��A���з�Ӧ�٣������ڳ����½��У��仯ѧ����ʽΪ ����ֻ���ڼ�������½��У���Ӧ��AӦΪ ��
(3)MnO2�ڷ�Ӧ�ٺͷ�Ӧ���е����÷ֱ��� �� ��
(4)�����Ƶ�F��ҺӦ���� �Է�ֹ��ת��ΪG������G��Һ�������ӵij����Լ��� ��ʵ������Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ͼ���漰�����ʾ�Ϊ��ѧ��ѧ�еij������ʣ�����CΪ��ɫ���嵥�ʣ�DΪ��ɫ���嵥�ʣ�EΪ�������ʣ�����Ϊ��������Ǵ�������ת����ϵ����Ӧ�����ɵ�ˮ����Ҫ���������ȥ����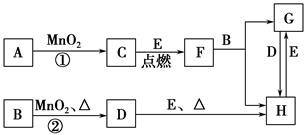
��1��д���й����ʵĻ�ѧʽ��F________��H________��
��2������Ӧ�����ڼ��������½��У���A��________������Ӧ�����ڳ��������½��У���A��________��
��3��д��B��MnO2���Ȼ��D�Ļ�ѧ����ʽ______________________________��
��4��B��ϡ��Һ��AgNO3��Һ��Ͽ��γɳ���AgX����֪Ksp��AgX����1.8��10��10����B��ϡ��Һ��AgNO3��Һ�������ϣ���B��Ũ��Ϊ2��10��4 mol��L��1�������ɳ�������AgNO3��Һ����СŨ��Ϊ________mol��L��1������AgX����Һ�еμ�KI��Һ���۲쵽��������______________________________�������ܹ�����ת����ԭ����_________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
A��B��X��Y��Ϊ��ѧ�εij������ʣ�����֮���ת����ϵ����ͼ��ʾ��
��ش��������⣺
��1����AΪ�������ʣ�BΪ�ǽ����������÷�Ӧ�Ļ�ѧ����ʽΪ ����д��������Ϊ26��A��һ�ֺ��ط��� ��
��2����AΪ�������ʣ�BΪ��ɫ���Ծ��壬��÷�Ӧ�Ļ�ѧ����ʽΪ ����д��A������������Һ��Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com