��2011?����ģ�⣩��̼�����Ҫ��ѧ�ɷ�ΪCeFCO
3��������ȡ����ϡ��Ԫ�ص���Ҫ����ԭ�ϣ���̼����ұ�����������Ѿ���չ��ʮ���֣�����һ����ȡ��Ĺ����������£�
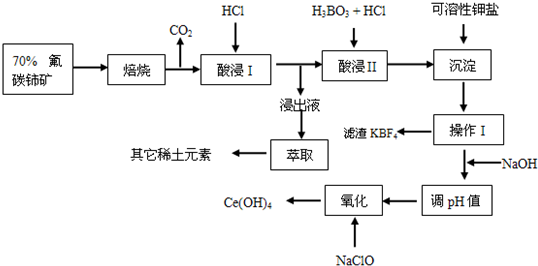
��֪�����պ������к�+4�۵��漰+3�۵�����ϡ������������I�Ľ���Һ�к�������+3�۵���
��ش���������
��1������ǰ����ʯ�����ϸ������Ŀ����
�������������ĽӴ����������Ӧ���ʣ����ԭ�ϵ�������
�������������ĽӴ����������Ӧ���ʣ����ԭ�ϵ�������
��
��2�����II���ļ���ת��Ϊ���ۣ�Ϊ�˱������������ķ���������ʽ��ʧ���ÿ����Լ��ν��ķ������������ȥ���÷�Ӧ�����ӷ���ʽΪ��
K++BF4-=KBF4��
K++BF4-=KBF4��
��3�������������У����I �л������������ɫ���壬��Ⱦ��������ʴ�豸��д����������ɫ��������ӷ���ʽ��
2Ce4++2Cl-=2Ce3++Cl2��
2Ce4++2Cl-=2Ce3++Cl2��
�����һ�ֽ���ķ�����
��H2SO4���
��H2SO4���
��
��4��ʵ�����н��в���I���ò����������ƣ�
©�����ձ���������
©�����ձ���������
���ڲ���I�����Һ�м���NaOH��Һ��Ϊ�˵�����ҺpHֵ���Ce��OH��
3���ⶨ����ҺpHֵ�IJ�����
˺��һСƬpH��ֽ���ڸ���ྻ�ı������ϣ��ò�����պȡ����Һ����pH��ֽ�����룬Ȼ�������ɫ���Աȣ�
˺��һСƬpH��ֽ���ڸ���ྻ�ı������ϣ��ò�����պȡ����Һ����pH��ֽ�����룬Ȼ�������ɫ���Աȣ�
��
��5��д����������Ļ�ѧ����ʽ��
2Ce��OH��3+NaClO+H2O=2Ce��OH��4+NaCl
2Ce��OH��3+NaClO+H2O=2Ce��OH��4+NaCl
�����л�ԭ������
NaCl
NaCl
��




