
��֪�����ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ����ᷢ����ˮ��Ӧ��

���з���ʽΪC9H8O2X2��XΪһδ֪Ԫ�أ�������M������һ�������·�������һϵ�з�Ӧ

�Իش��������⣺
��1��XΪ ����Ԫ�� ���ţ���
��2��A�������Ĺ�����Ϊ ������ת������������������Ӧ�Ĺ��� ���������֣���M��NaOH��Һ���ȷ�Ӧ������������ ��Ӧ��
��3��M�Ľṹ��ʽΪ ��
��4��д�����з�Ӧ�Ļ�ѧ���̣�
��B��D�� ��
�� M��NaOH��Һ������Ӧ�Ļ�ѧ����ʽ ��
��E��������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��ÿ��2�֣���16�֣�
��1��Br
��2��ȩ�� ����-CHO�����Ȼ���-COOH������1����1�֣���2�֣��� 4�� �� ȡ����Ӧ����ˮ�ⷴӦ��
��3��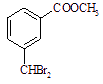
��4����2CH3OH��O2![]() 2HCHO��2H2O
2HCHO��2H2O
![]() ��
�� +3NaOH 2NaBr +CH3OH+
+3NaOH 2NaBr +CH3OH+
 +H2O��ע�������������ƽ��С���ӵȵĹ淶��
+H2O��ע�������������ƽ��С���ӵȵĹ淶��
��HCOOH��2Ag(NH3)2OH![]() (NH4)2CO3��2Ag����2NH3����H2O����������д��CO2��H2CO3��NH4HCO3��HOCOONH4�����ԣ�
(NH4)2CO3��2Ag����2NH3����H2O����������д��CO2��H2CO3��NH4HCO3��HOCOONH4�����ԣ�


 ��ڽ��ȫ������ϵ�д�
��ڽ��ȫ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?���ݶ�ģ����֪�����ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ���Ҫ������ˮ��Ӧ��
��2011?���ݶ�ģ����֪�����ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ���Ҫ������ˮ��Ӧ��




�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
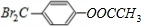
 ���з���ʽΪC9H8O2Br2������M����һ�������¿ɷ�����ͼ��ʾһϵ�з�Ӧ����֪�л���A����Է�������Ϊ60����
���з���ʽΪC9H8O2Br2������M����һ�������¿ɷ�����ͼ��ʾһϵ�з�Ӧ����֪�л���A����Է�������Ϊ60����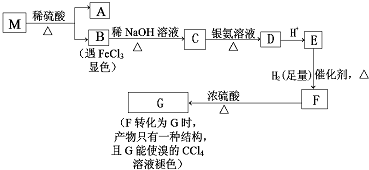

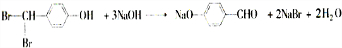
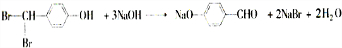
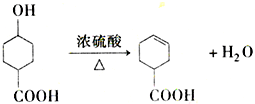

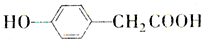
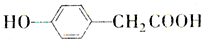
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ������ѧ2009����������λ�ѧ�����¿��Ծ�һ ���ͣ������
��֪�����ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ����ᷢ����ˮ��Ӧ��
���з���ʽΪC9H8O2X2��XΪһδ֪Ԫ�أ�������M������һ�������·�������һϵ�з�Ӧ
�Իش��������⣺
��1��XΪ ����Ԫ�� ���ţ���
��2��A�������Ĺ�����Ϊ ������ת������������������Ӧ�Ĺ��� ���������֣���M��NaOH��Һ���ȷ�Ӧ������������ ��Ӧ��
��3��M�Ľṹ��ʽΪ ��
��4��д�����з�Ӧ�Ļ�ѧ���̣�
��B��D�� ��
�� M��NaOH��Һ������Ӧ�Ļ�ѧ����ʽ ��
��E��������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ��ɽ��ʡ�߿�һ�ָ�ϰ�ο��Ի�ѧ�Ծ��������棩 ���ͣ������
��12�֣���֪�����ǻ�ͬʱ����ͬһ̼ԭ���ϵĽṹ�Dz��ȶ��ģ���Ҫ������ˮ��Ӧ��


���з���ʽΪC9H8O2X2��XΪһδ֪Ԫ�أ�������M������һ�������·�������һϵ�з�Ӧ
�Իش��������⣺
�� XΪ ����Ԫ�� ���ţ���
��A�������Ĺ�����Ϊ ������ת������������������Ӧ�Ĺ��� ���������֣���M��NaOH��Һ���ȷ�Ӧ������������ ��Ӧ��
�� M�Ľṹ��ʽΪ ��
��д�����з�Ӧ�Ļ�ѧ���̣�
��B��D ��
��D��һ�������·����Ӿ۷�Ӧ�� ��
��E��������Һ��Ӧ�Ļ�ѧ����ʽ�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com