
����Ŀ��þ����Ͻ���һ����;�ܹ�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ���Ҫ����:
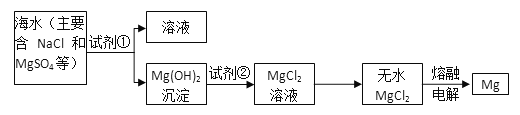
�Իش��������⣺
��1��Ϊ��ʹMgSO4ת��ΪMg(OH)2���Լ��ٿ���ѡ��__________________________��
��2�������Լ��ٺ��ܹ�����õ�Mg(OH)2�����ķ�����____________________��
��3���Լ��ڿ���ѡ��_______����Ӧ�����ӷ���ʽΪ��__________________________��
��4����ˮMgCl2������״̬�£�ͨ�������Mg��Cl2��д���÷�Ӧ�Ļ�ѧ����ʽ________________________��
���𰸡� ������ʯ��������NaOH ���� ���� Mg(OH)2��2H��===Mg2����2H2O MgCl2(����)![]() Mg+Cl2��
Mg+Cl2��
��������(1)MgSO4��NaOH��ʯ���鷴Ӧ������Mg(OH)2�����ӷ���ʽΪMg2++2OH-�TMg(OH)2����ҪʹMgSO4��ȫת��Ϊ�����������Լ�����Ӧ�������ʴ�Ϊ��������ʯ�����NaOH��
(2)��Mg(OH)2����������ˮ�����ù��˵ķ������룬�ʴ�Ϊ�����ˣ�
(3)Mg(OH)2��HCl��Ӧ������MgCl2��H2O������ʽΪ��Mg(OH)2+2HCl=MgCl2+2H2O���ʴ�Ϊ������(HCl)��Mg(OH)2+2H+=Mg2++2H2O��
(4)��ˮMgCl2������״̬�£�ͨ�������Mg��Cl2��MgCl2(����)![]() Mg+Cl2�����ʴ�Ϊ��MgCl2(����)
Mg+Cl2�����ʴ�Ϊ��MgCl2(����)![]() Mg+Cl2����
Mg+Cl2����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����(N2H4)��һ�ָ���ȼ�ϣ��ڹ�ҵ��������;�㷺��
��1����(N2H4)Ҳ����NO2������Ӧ�����������Ⱦ��������д����Ӧ����ʽ��____________________��
��2���������백�����ƣ�������ˮ���ɷ������µ�����̣�
N2H4 + H2O ![]() N2H5+ + OH- I N2H5+ +H2O
N2H5+ + OH- I N2H5+ +H2O ![]() N2H62+ + OH- II
N2H62+ + OH- II
�� �����£�ijŨ��N2H6Cl2��Һ��pHΪ4�������Һ����ˮ���������c(H+)Ϊ__________��
�� ��֪����ͬ�����¹���I�Ľ��г̶ȴ���N2H5+��ˮ��̶ȡ������£���0.2 mol/L N2H4��Һ��0.1 mol/L HCl��Һ�������ϣ����ʱ��Һ��_________�ԣ���Һ��N2H5+��Cl-��OH-��H+��N2H4Ũ���ɴ�С��˳��Ϊ___________________��

��3���º������ڲ�ͬ�¶Ⱥʹ������������ɲ�ͬ�����ͼ����
�¶Ƚϵ�ʱ��Ҫ������Ӧa��N2H4(g)+O2(g)![]() N2(g)+2H2O(g)
N2(g)+2H2O(g)
�¶Ƚϸ�ʱ��Ҫ������Ӧb��N2H4(g)+2O2(g)![]() 2NO(g)+2H2O(g)
2NO(g)+2H2O(g)
������Ӧb��1000��ʱ��ƽ�ⳣ��ΪK1��1100��ʱ��ƽ�ⳣ��ΪK2����K1__________K2�����>������<����=����
��ij�¶��£��ݻ��̶����ܱ������У�����������˵����Ӧa�ﵽƽ�����_______________��
A��v(N2)=v(N2H4)
B��c(N2H4):c(O2):c(N2)=1:1:1
C�����������ܶȲ��ٸı�
D����ϵ��ѹǿ���ٷ����仯
E����������ƽ����Է����������ٷ����仯
��1000�棬��Ӧb�ﵽƽ��ʱ�����д�ʩ��ʹ������![]() ������_______��
������_______��
A�����������³���He B������������� C�������������³���N2H4 D��ʹ�ô���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij�����ŷŵĹ�ҵ��ˮ�п��ܺ���K+��Ag+��NH4+��Mg2+��SO42-��Cl-��NO3-��HCO3-�����ӣ�������ˮ�����������ԣ�����ȷ���ó���ˮ�п϶������е�����������
A��Ag+��K+��NO3-��HCO3- B��K+��NH4+��NO3-��SO42-
C��Ag+��NH4+��Mg2+��HCO3- D��K+��Mg2+��SO42-��Cl-
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������Ȫɽɭ�ֹ�����Ϊ��Ȼ���ɣ���ԭ���ǿ����е����ɵ��Ӹ����ڷ��ӻ�ԭ�����γɿ��������ӣ�����Ϊ������ά��������O2������һ�ֿ��������ӣ���Ħ������Ϊ
A. 33 g B. 32 g C. 33 g��mol��1 D. 32 g��mol��1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ú�ˮ������ȡ�Ȼ��ơ��塢��Ȳ�Ʒ�����������������£�
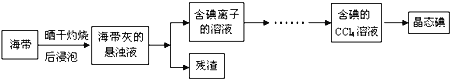
��1��CCl4����___________����______________����(��ѡ����ԡ��Ǽ��ԡ�)��
��2�����պ���ʱ����Ҫ���żܡ��ƾ������������⣬����Ҫ����Ҫ������____________��
��3��Ϊ���õ����ʵ�飬��������ʱ�������һ�������������Ƴ�ֻ�Ϻ����������˲���Ŀ����___________________________________��
��4����ʢ�е�������Һ�м���CCl4����ˮ��______(����ϡ����¡�)����Ϻ�ɫ�������ˮ�ӹ�����CCl4������Ϻ�ɫ�����ɫ�����������б�����������__________________��д������ƽCCl4�����Ϻ�ɫ�����ɫ�Ļ�ѧ��Ӧ����ʽ��__________
![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ij�¶�ʱ����һ��10 L�ĺ��������У�X��Y��Z��Ϊ���壬�������ʵ����ʵ�����ʱ��ı仯������ͼ��ʾ��

����ͼ��������գ�
��1���÷�Ӧ�Ļ�ѧ����ʽΪ_________________________________��
��2����Ӧ��ʼ��2 min��������Z��ʾ��ƽ����Ӧ����Ϊ____________________��
���ں��º��ݵ��ܱ������У����������������ٷ����仯ʱ���ٻ�������ѹǿ���ڻ��������ܶȣ��ۻ������������ʵ������ܻ�������ƽ����Է����������ݻ���������ɫ��
��1��һ����֤��2SO2(g)+O2(g) ![]() 2SO3(g)�ﵽƽ��״̬����__________������ţ���ͬ����
2SO3(g)�ﵽƽ��״̬����__________������ţ���ͬ����
��2��һ����֤��I2(g) +H2(g) ![]() 2HI(g)�ﵽƽ��״̬����___________��
2HI(g)�ﵽƽ��״̬����___________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���йش����Ĵ�������������Դӡ��Ҵ�������ʵ�顱�õ�һЩ��ʶ,ij��ʦ�������ͼ��ʾװ��(�г�װ�õ���ʡ��),��ʵ�����Ϊ:�Ȱ�ͼ��װ��װ��,�رջ���a��b��c,��ͭ˿���м䲿�ּ���Ƭ��,Ȼ�����a��b��c,ͨ�����ƻ���a��b,���н���(��Ъ��)��ͨ������,������M���۲쵽���Ե�ʵ�������Իش���������:

��1��A�з�����Ӧ�Ļ�ѧ����ʽ:_____________________________________,
B������:_____________________;C����ˮ������:________________________________��
(2)M��������Ӧ�Ļ�ѧ����ʽΪ________________________________��
(3)��M���пɹ۲쵽������:_________________________________��
(4)ʵ�����һ��ʱ���,��������ƾ���,��Ӧ__(��ܡ����ܡ�)��������,��ԭ����______________________________________________________________________��
(5)��֤�Ҵ���������Ļ�ѧ������____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����������У��������л�����ǣ� ��
A. ����(CH3CH2CH3)B. ����(CH3COOH)
C. �軯��(NaCN)D. ��Ȳ(C2H2)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͭ����һ����ϡ���������������Һ�У���ַ�Ӧ����Һ����ɫ������ɫ����������������������ײ����������壬��ù�������ǣ� ��
A.Cu
B.S
C.CuS
D.Cu2S
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com