
��֪ij�¶��£�N2(g)��3H2(g)![]() 2NH3(g)��H����92.4 kJ��mol��1�����¡��������ݻ���ͬ�������ܱ�����A��B��A��ͨ��1 mol N2��3 mol H2��B��ͨ��0.5 mol N2��1.5 mol H2����Ӧһ��ʱ���A��B�о��ﵽƽ��״̬�������ж���ȷ���� (����)
2NH3(g)��H����92.4 kJ��mol��1�����¡��������ݻ���ͬ�������ܱ�����A��B��A��ͨ��1 mol N2��3 mol H2��B��ͨ��0.5 mol N2��1.5 mol H2����Ӧһ��ʱ���A��B�о��ﵽƽ��״̬�������ж���ȷ���� (����)
A��A��N2ת����С��B��2�� B��A�з���С��B�е�2��
C��A�з��ȴ���B�е�2�� D��A��N2Ũ�ȴ���B��2��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��2011?�Ͼ���ģ����Ч��ת�����ɼ�������β���е���Ҫ��Ⱦ�CO��NOx�ȣ������ַ�Ӧ���£�
��2011?�Ͼ���ģ����Ч��ת�����ɼ�������β���е���Ҫ��Ⱦ�CO��NOx�ȣ������ַ�Ӧ���£��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A.A������92.4 kJ B.A�������仯ֵ����B�е�2��
C.B�з���46.2 kJ D.A�������仯ֵ����B�е�2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��0103 �¿��� ���ͣ���ѡ��
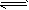 2NH3(g)����H=��92.4 kJ/mol�����к��¡������������ͬ�������ܱ�����A��B��A��ͨ��1mol N2��3mol H2��B��ͨ��0.5mol N2��1.5mol H2����Ӧһ��ʱ��������������ѹǿ�����ٷ����仯�������ж���ȷ����
2NH3(g)����H=��92.4 kJ/mol�����к��¡������������ͬ�������ܱ�����A��B��A��ͨ��1mol N2��3mol H2��B��ͨ��0.5mol N2��1.5mol H2����Ӧһ��ʱ��������������ѹǿ�����ٷ����仯�������ж���ȷ�����鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com