
�л���A�����µ�ת����ϵ:
��1��A�ķ���ʽΪ ��1molA�����Ժ� molH2��Ӧ��
��2��������C�Ľṹ��ʽΪ ���京�������ŵ������� ��
��3��A����������Ӧ�Ļ�ѧ����ʽΪ ��
��4������˵����ȷ���� ������ĸ����
a����Ӧ�ٵķ�Ӧ����Ϊ�ӳɷ�Ӧ
b��A��һ�ȴ�����2��
c��B�ܸ�Na��Ӧ�ų�H2������FeCl3��Һ����ɫ
d��1molD������NaOH��Һ��Ӧ��������2molNaOH
��5��C��ŨH2SO4�ͼ��ȵ������£���������D�⣬��������һ�ָ߷��ӻ�����E�� E�Ľṹ��ʽΪ ��
��16�֣���1��C8H8O��2�֣� 4 ��2�֣�
��2��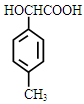 �ǻ����Ȼ�
�ǻ����Ȼ�
��3��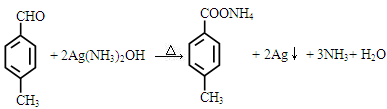
��4��ad
��5��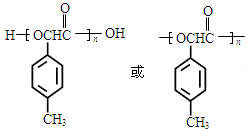
���������������1���ɽṹ��ʽ�ƶϣ�A��3��ԭ���ţ���CHO����C6H4������CH3�����ɣ���A�ķ���ʽΪC8H8O������1mol�����Ժ�3molH2��1mol��ȩ���Ժ�1molH2�����ӳɷ�Ӧ��ԭ��Ӧ�����ݽṹ������������ԭ���ƶϣ�1molA�����Ժ�4mol H2�����ӳɷ�Ӧ��ԭ��Ӧ����2��������֪��Ӧ��Ϣ��ȣ������ƶ�B�У�CNҲ��ת��Ϊ��COOH������ԭ���ű��ֲ��䣬�ɷ�Ӧ���������ƶ�B��ת������C�Ľṹ��ʽ��C�������ֹ����ţ��ֱ����ǻ����Ȼ�����3����ȩ��������ӦΪCH3CHO+2Ag(NH3)2OH CH3COONH4+2Ag��+3NH3+H2O������AҲ����ȩ�����ɴ˿��Է�д��A������������Ӧʽ����4���Ա�A��B�Ľṹ��ʽ��֪����Ӧ��Ϊ̼��˫����H��CN�����ļӳɷ�Ӧ����a��ȷ�������ϵ�ȩ������λ�����λ�ã����ݵ�Ч�ⷨ�ƶϣ�A���Ӻ���4����ԭ�ӣ����A��һ�ȴ��ﳬ��2�֣���b����B�����ǻ������������ý����Ʒ����û���Ӧ���ų������������ǻ�û���뱽��ֱ�����������Ƿ��ǻ��������зӵ����ʣ����ܷ�����ɫ��Ӧ����c����D����2��������������ṹ��֪����1molD��2molH2O����Ӧ����������2mol�Ȼ���2mol���ǻ���ǰ���ܵ����H+�����߲����ܵ����H+������ƶ�1molD���������2molNaOH����d��ȷ����5��C�����ǻ����ᣬ���������Ҵ�����������Ӧ��ԭ����֪��nmolC��ȥ(n��1)mol�Ȼ���(n��1)mol�ǻ�������1mol�߾�����(n��1)molH2O������nmolC��ȥnmol�Ȼ���nmol�ǻ�������1mol�߾�����nmolH2O���ɴ˿�����д�߾���E�Ľṹ��ʽ��
CH3COONH4+2Ag��+3NH3+H2O������AҲ����ȩ�����ɴ˿��Է�д��A������������Ӧʽ����4���Ա�A��B�Ľṹ��ʽ��֪����Ӧ��Ϊ̼��˫����H��CN�����ļӳɷ�Ӧ����a��ȷ�������ϵ�ȩ������λ�����λ�ã����ݵ�Ч�ⷨ�ƶϣ�A���Ӻ���4����ԭ�ӣ����A��һ�ȴ��ﳬ��2�֣���b����B�����ǻ������������ý����Ʒ����û���Ӧ���ų������������ǻ�û���뱽��ֱ�����������Ƿ��ǻ��������зӵ����ʣ����ܷ�����ɫ��Ӧ����c����D����2��������������ṹ��֪����1molD��2molH2O����Ӧ����������2mol�Ȼ���2mol���ǻ���ǰ���ܵ����H+�����߲����ܵ����H+������ƶ�1molD���������2molNaOH����d��ȷ����5��C�����ǻ����ᣬ���������Ҵ�����������Ӧ��ԭ����֪��nmolC��ȥ(n��1)mol�Ȼ���(n��1)mol�ǻ�������1mol�߾�����(n��1)molH2O������nmolC��ȥnmol�Ȼ���nmol�ǻ�������1mol�߾�����nmolH2O���ɴ˿�����д�߾���E�Ľṹ��ʽ��
���㣺�����л��ϳɺ��ƶϴ��⣬�漰����ʽ���ṹ��ʽ����ѧ����ʽ����д���Լ������������ʵ��жϵ�֪ʶ��


 һ���㶨ϵ�д�
һ���㶨ϵ�д� ��У��ҵ��ϵ�д�
��У��ҵ��ϵ�д� ���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
���ɶ���ܲ��¿�ֱͨ��Уϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л��߷��Ӳ���I��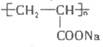 ����һ�ֱ�ˮ������������ˮ������ǿ���ܷ�����ˮ����ˮ�����ǰ�������Ϊũҵ�ϡ���ˮ�⡱����������A����ϩ��ͨ�����·�Ӧ�Ƶã�����D�Ľṹ��ʽΪ��
����һ�ֱ�ˮ������������ˮ������ǿ���ܷ�����ˮ����ˮ�����ǰ�������Ϊũҵ�ϡ���ˮ�⡱����������A����ϩ��ͨ�����·�Ӧ�Ƶã�����D�Ľṹ��ʽΪ��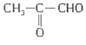 ��F�Ľṹ��ʽΪ��
��F�Ľṹ��ʽΪ��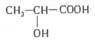 ����ش�
����ش�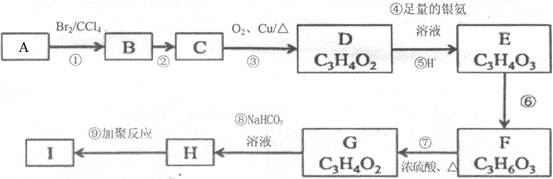
��1��B��H�Ľṹ��ʽ��B ��H ��
��2����Ӧ�ڵ����� ����Ӧ�ߵ����� ��
��3����Ӧ�ܵĻ�ѧ����ʽΪ ��
��4��M��һ����2��F������Ũ����ͼ��������·�Ӧ���ɵĻ������÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5�������й�D(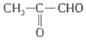 )��˵��������� ��
)��˵��������� ��
���ܷ���������Ӧ
��1Ħ��D��H2�ӳ�ʱ�������2molH2
��1Ħ��D���ȼ��������Ҫ����2mol O2
����ʹ���Ը��������Һ��ɫ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
��֪��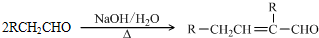
ˮ������EΪ�������ռ������������Ʒ�ɹ˪��E��һ�ֺϳ�·�����£�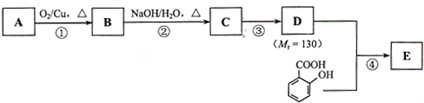
��ش��������⣺
��1��һԪ��A��������������ԼΪ21.6%����A�ķ���ʽΪ ���ṹ������ʾAֻ��һ������A������Ϊ ��
��2��B�������Ƶ�Cu(OH)2������Ӧ���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��3��C�� �ֽṹ����һ��ȡ��������C�����������ţ���ʹ�õ��Ⱥ�˳��д�������Լ� ��
��4���ڢ۵ķ�Ӧ����Ϊ ��D���������ŵ�����Ϊ ��
��5��д��ͬʱ��������������ˮ��������ͬ���칹��Ľṹ��ʽ�� ��
A�������к���6��̼ԭ����һ������
B�����������������Ű���ˮ������еĹ�����
��6���ڢܲ��ķ�Ӧ����Ϊ ��д��E�Ľṹ��ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�����������͡����ס���Ч������ʧ��ҩ�����ڶ�������ʧ���ķ��Ρ��ṹΪ��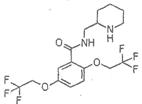 �������������ᣨ
�������������ᣨ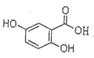 ��Ϊԭ�Ϻϳɣ��ϳɵķ�������ͼ��
��Ϊԭ�Ϻϳɣ��ϳɵķ�������ͼ��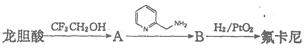
�ش��������⣺
��1��д��һ���������к��еĺ�������������______________��
��2��A�Ľṹ��ʽ��____________________________________��
��3��A��B�ķ�Ӧ������______________��
��4����������״���Ӧ����������������仯ѧ����ʽ��______________��
��5���������������������������ͬ���칹�干��_______�֣�
�ٱ�������3��ȡ����������2��Ϊ�ǻ����ڱ�����ֻ�������⣻���������ࡣ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�ԶԼӣ�A��Ϊ��ʼԭ�ϣ�ͨ��һϵ�з�Ӧ�ϳ��л���E�ĺϳ�·�����£�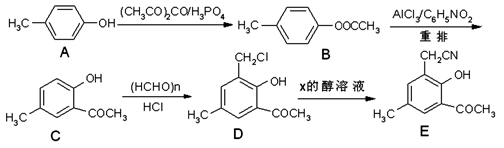
��1��C�ķ���ʽΪ ��A�ĺ˴Ź�������ͼ���� ���塣
��2��A��B�ķ�Ӧ����Ϊ ��
��3��д��D������NaOH��ˮ��Һ��Ӧ�Ļ�ѧ����ʽ�� ��
��4��д��ͬʱ��������������D��ͬ���칹��Ľṹ��ʽ�� �� ����д���֣���
����������������������ĸ�ȡ�����ұ����ϵ�һȡ������ֻ��һ�֣�
������Na2CO3��Һ��Ӧ�ų����塣
��5����֪��R-CN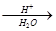 R-COOH��E������������ˮ���IJ�����һ�������¿�����
R-COOH��E������������ˮ���IJ�����һ�������¿�����
F��C11H10O3����д��F�Ľṹ��ʽ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�л���A��G��ת����ϵ����ͼ��ʾ������A��һԪ��״�������˴Ź���������ֻ��1���壻F�ĺ˴Ź�����������3���壬�������Ϊ2��2��3��G��һ�ֺϳ�����֬��һ����Ҫԭ�ϡ�
��֪��(RΪ����) ��
��RCOOH RCH2OH
RCH2OH
��ش��������⣺
��1��C�����������ŵ������� ����Ӧ�ܵķ�Ӧ������___________________��
��2����E��һ�������·������۷�Ӧ���ɸ߷��ӻ����д���������ֵĽṹ��ʽ��
_____________________________��_____________________________��
��3����Ӧ�ڵĻ�ѧ����ʽΪ ��
��4����Ӧ�Ļ�ѧ����ʽΪ________________________________________________________��
��5���л���Y��E��Ϊͬ���칹�壬���Ҿ�����ͬ�Ĺ������������Ŀ��д�����з���������Y�Ľṹ��ʽ��_____________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��ƶ���
�����������Ƹΰ��ķ�չ��ȥ���������е��������ƵõĻ������ȥ���������Ծ�����Ӧ����Ч����ϳ�·�����£�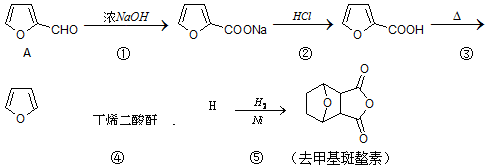
��֪���� 2HCHO+NaOH�� CH3OH+HCOONa
�� (Diels-Alder ��Ӧ)
(Diels-Alder ��Ӧ)
�۵�ÿ��1��3-����ϩ������һ�������������ӳɷ�Ӧʱ�������ֲ�� CH2ClCH=CHCH2Cl��CH2ClCHClCH=CH2��
��ش��������⣺
��1������A�к��еĺ������������Ʒֱ�Ϊ ���ڢٲ���Ӧ�л�������һ����˲���Ľṹ��ʽΪ ��
��2��д��H�Ľṹ��ʽ ���ڢݲ���Ӧ�ķ�Ӧ������ ��
��3��ȥ����������X��Ϊͬ���칹�塣X����FeCl3��Һ������ɫ��Ӧ,����Na2CO3��Һ��Ӧ�������壬����˴Ź���������4�����շ塣д����������������X��һ�ֽṹ��ʽ ��
��4����������й���Ϣ��д����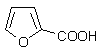 �Ʊ���ϩ������
�Ʊ���ϩ������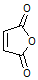 �ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�
�ĺϳ�·������ͼ�����Լ���ѡ�����ϳ�·������ͼʾ�����£�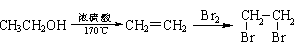
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�߶��ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ�������£����淴Ӧ��N2(g)+3H2(g)  2NH3(g)����H<0���ﵽƽ��ʱ���������ı������������й������������( )
2NH3(g)����H<0���ﵽƽ��ʱ���������ı������������й������������( )
A���Ӵ���V����V�涼�����仯�ұ仯�ı������
B����ѹ��V����V�涼������V������������V��������
C�����£�V����V�涼��С����V����С����С��V���С����
D�����������V����V�涼������V������������V��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014-2015ѧ�����ʡ�߶���һѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ���¶������£��ס��������ݻ���ȵĺ����ܱ������о��������·�Ӧ��3A��g����B(g)  xC(g)��D(s)�������ͨ��6molA��2 molB��������ͨ��1.5molA��0.5molB��3molC��2molD����Ӧһ��ʱ��ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ20%�����������в���ȷ����
xC(g)��D(s)�������ͨ��6molA��2 molB��������ͨ��1.5molA��0.5molB��3molC��2molD����Ӧһ��ʱ��ﵽƽ�⣬��ʱ��üס�����������C�����������Ϊ20%�����������в���ȷ����
A����ƽ��ʱ���ס�����������A�����ʵ�������ȣ���x=4
B��ƽ��ʱ���ס�����������A��B�����ʵ���֮�����
C��ƽ��ʱ����A���������Ϊ40%
D����ƽ��ʱ�������е�ѹǿ����ȣ�����������ѹǿ֮��Ϊ8��5
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com