
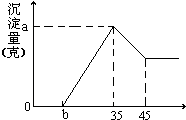
 ȷ��ȡ6g������Al2O3��Fe2O3��SiO2����Ʒ������ʢ��100mL ϡH2SO4��Һ���ձ��У���ַ�Ӧ�����ȥ������������Һ�м���10mol/L��NaOH��Һ�����������������ͼ����NaOH��Һ�������mL����ͼ��ʾ���Իش��������⣺
ȷ��ȡ6g������Al2O3��Fe2O3��SiO2����Ʒ������ʢ��100mL ϡH2SO4��Һ���ձ��У���ַ�Ӧ�����ȥ������������Һ�м���10mol/L��NaOH��Һ�����������������ͼ����NaOH��Һ�������mL����ͼ��ʾ���Իش��������⣺ =0.05mol��
=0.05mol�� n��NaOH��=
n��NaOH��= ��0.35mol=0.175mol��
��0.35mol=0.175mol��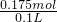 =1.75mol/L��
=1.75mol/L�� ���㣮
���㣮


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ȷ��ȡ6g������Al2O3��Fe2O3��SiO2����Ʒ������ʢ��100mL ϡH2SO4��Һ���ձ��У���ַ�Ӧ�����ȥ������������Һ�м���10mol/L��NaOH��Һ�����������������ͼ����NaOH��Һ�������mL����ͼ��ʾ���Իش��������⣺
ȷ��ȡ6g������Al2O3��Fe2O3��SiO2����Ʒ������ʢ��100mL ϡH2SO4��Һ���ձ��У���ַ�Ӧ�����ȥ������������Һ�м���10mol/L��NaOH��Һ�����������������ͼ����NaOH��Һ�������mL����ͼ��ʾ���Իش��������⣺�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1�����к�Fe2O380%�ij�����100t�������Ͽ�ұ����Fe95%���������ٶ֣�
��1�����к�Fe2O380%�ij�����100t�������Ͽ�ұ����Fe95%���������ٶ֣��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��16�֣���������Ҫ�ɷ�ΪAl2O3��������SiO2��Fe2O3���ǹ�ҵ���Ʊ�����������Ҫԭ�ϡ���ҵ����ȡ�������Ĺ����������£�
��1������A��B�ijɷֱַ��� �� ��
��2��������е��Լ�a�� ��
��3����д��������з�����Ӧ�����ӷ�ʽ ��
��4����ʵ����ģ������ʵ������У���Ҫ�õ�һ��Ũ�ȵ�������Һ��������250mi����������Һʱ��ijͬѧת����Һ�IJ�����ͼ��ʾ��ͼ�е���Ҫ�����ǣ�
�� ��
�� ��
��4��ȷ��ȡ6g��������Ʒ������100mL������Һ����ַ�Ӧ������Һ�м���10 mol��L-1�Լ�a����Һ����������������������Լ�a�������ϵ��ͼ��ʾ��������������Һ�����ʵ���Ũ��Ϊ ����Ʒ��Al2O3�İٷֺ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010��ɽ��ʡ���һ�и�����ѧ�ڵ�һ���¿�����ѧ�� ���ͣ������
��16�֣���������Ҫ�ɷ�ΪAl2O3��������SiO2��Fe2O3���ǹ�ҵ���Ʊ�����������Ҫԭ�ϡ���ҵ����ȡ�������Ĺ����������£�
��1������A��B�ijɷֱַ��� �� ��
��2��������е��Լ�a�� ��
��3����д��������з�����Ӧ�����ӷ�ʽ ��
��4����ʵ����ģ������ʵ������У���Ҫ�õ�һ��Ũ�ȵ�������Һ��������250mi����������Һʱ��ijͬѧת����Һ�IJ�����ͼ��ʾ��ͼ�е���Ҫ�����ǣ�
�� ��
�� ��
��4��ȷ��ȡ6g��������Ʒ������100mL������Һ����ַ�Ӧ������Һ�м���10 mol��L-1�Լ�a����Һ����������������������Լ�a�������ϵ��ͼ��ʾ��������������Һ�����ʵ���Ũ��Ϊ ����Ʒ��Al2O3�İٷֺ���Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010-2011ѧ��㶫ʡ÷���и�����ѧ����ĩ���ԣ����ۣ���ѧ���� ���ͣ������
��������Ҫ�ɷ�ΪAl2O3��������SiO2��Fe2O3���ǹ�ҵ���Ʊ�����������Ҫԭ�ϡ���ҵ����ȡ�������Ĺ����������£�

��1������ A��B�ijɷֱַ��� �� ��
��2��������е��Լ�a�� ��
��3����д��������з�����Ӧ�����ӷ�ʽ ��
��4��ȷ��ȡ6g��������Ʒ������100mL������Һ����ַ�Ӧ������Һ�м���10 mol��L-1�Լ�a����Һ����������������������Լ�a�������ϵ����ͼ��ʾ��������������Һ�����ʵ���Ũ��Ϊ ����Ʒ��Al2O3�İٷֺ���Ϊ ��

�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com