
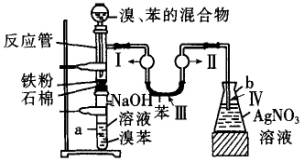
�밴Ҫ��ش�
��1���йػ�ѧ����ʽΪ__________________________________________��
��2���ڵ�����Һ�����Ӻ��ܹ۲쵽�������ǣ�
����______________������______________������_____________������____________��
��3����2 min��3 min������a�ܵײ��õ�____________��
��4��Fe�۵�������_____________��NaOH��Һ��������_____________��U�ι��б���������_____________��AgNO3��Һ��������_____________��
��5��a�ܴ���֧�ܣ�������Ϊ_____________��Ϊ��ǿ�����ã�������֧�ܿڴ����еIJ�����_____________��
 ��һ����ͬ���ɽ�����ϵ�д�
��һ����ͬ���ɽ�����ϵ�д� ������Ӧ���ϵ�д�
������Ӧ���ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
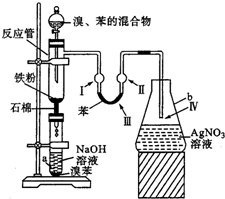 ijѧ���С��������屽����ȡװ�ã���ͼ��ʾ������д���пհף�
ijѧ���С��������屽����ȡװ�ã���ͼ��ʾ������д���пհף�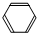 +Br2
+Br2| �廯�� |
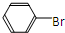 +HBr��
+HBr�� +Br2
+Br2| �廯�� |
 +HBr��
+HBr���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�058
��������屽����ȡװ������ͼ��ʾ��
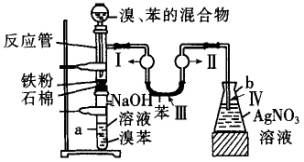
�밴Ҫ��ش�
��1���йػ�ѧ����ʽΪ__________________________________________��
��2���ڵ�����Һ�����Ӻ��ܹ۲쵽�������ǣ�
����______________������______________������_____________������____________��
��3����2 min��3 min������a�ܵײ��õ�____________��
��4��Fe�۵�������_____________��NaOH��Һ��������_____________��U�ι��б���������_____________��AgNO3��Һ��������_____________��
��5��a�ܴ���֧�ܣ�������Ϊ_____________��Ϊ��ǿ�����ã�������֧�ܿڴ����еIJ�����_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
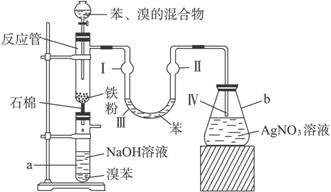
(1)��ӦʽΪ_____________________________________________��
(2)�ڵ�����Һ�����Ӻ��ܹ۲쵽�������ǣ�
(��)��______________________��
(��)��______________________��
(��)��______________________��
(��)��______________________��
(3)��2 min��3 min������a�ܵײ��õ�_________��
(4)NaOH��������______________________��
U�ι��б���������____________________��
AgNO3��Һ��������____________________��
(5)a�ܴ���֧�ܣ�������Ϊ___________________��
Ϊ��ǿ�����ã�������֧�ܿڴ����еIJ�����______________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������屽����ȡװ������ͼ��ʾ���밴Ҫ������������⣺
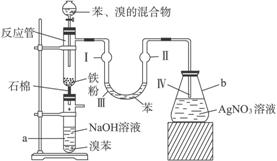
(1)��ӦʽΪ_____________________________________________��
(2)�ڵ�����Һ�����Ӻ��ܹ۲쵽�������ǣ�
(��)��______________________��
(��)��______________________��
(��)��______________________��
(��)��______________________��
(3)��2 min��3 min������a�ܵײ��õ�_________��
(4)NaOH��������______________________��
U�ι��б���������____________________��
AgNO3��Һ��������____________________��
(5)a�ܴ���֧�ܣ�������Ϊ___________________��
Ϊ��ǿ�����ã�������֧�ܿڴ����еIJ�����______________________��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com