
��I2��KI��Һ�д�������ƽ�⣺l2(aq)+I-(aq)FI3-(aq)��ijI2��KI�����Һ�У�I3-�����ʵ���Ũ��c(I3-)���¶�T�Ĺ�ϵ��ͼ��ʾ(�������κ�һ�㶼��ʾƽ��״̬)��
����˵������ȷ����
A����ӦI2(aq)+I-(aq)I3-(aq)�ġ�H<O
B�����¶�ΪT1��T2ʱ����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2����K1>K2
C������Ӧ���е�״̬Dʱ��һ����![]() >
>
D��״̬A��״̬B��ȣ�״̬A��C(I2)��
 ��һ������Ԫͬ�����ؾ�ϵ�д�
��һ������Ԫͬ�����ؾ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(9��)��֪�����������������·�Ӧ��ϵ��
��KBrO3�ܽ�KI������I2��KlO3���䱾������ԭΪBr2
2BrO3����10I����12H��===5I2��Br2��6H2O 6BrO3����5I����6H��===5IO3����3Br2��3H2O
��Br2�ܽ�I������ΪI2
Br2��2I��===2Br����I2
��KIO3�ܽ�I������ΪI2��Ҳ�ܽ�Br��������Br2���䱾������ԭΪI2
IO3����5I����6H��===3I2��3H2O 2IO3����10Br����12H��===I2��5Br2��6H2O
(1)��������Ӧ�漰����������������ǿ����________��
(2)��KI��KBr�Ļ����Һ�У����������KBrO3������������Ϊ________����ԭ����Ϊ________��
(3)����1mol KI��������Һ�м���KBrO3��Һ����Ӧ���Ԫ��ֻ������I2�У���Ԫ��ֻ������Br���У������KBrO3�����ʵ���Ϊ________��
(4)��6mL 0.4 mol��L��1 KBrO3��Һ��10 mL 0.4 mol��L��1KI��Һ��ϡH2SO4�л�ϡ�д��������Ӧ�����ӷ���ʽ______________________________________________________��
(4)10I����6BrO3����12H��===3Br2��3I2��4IO3����6H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�������ѧ�ڻ�ѧһ�ָ�ϰ���Ӻ�ˮ�л�û�ѧ���ʡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ������
(9��)��֪�����������������·�Ӧ��ϵ��
��KBrO3�ܽ�KI������I2��KlO3���䱾������ԭΪBr2
2BrO3����10I����12H��===5I2��Br2��6H2O 6BrO3����5I����6H��===5IO3����3Br2��3H2O
��Br2�ܽ�I������ΪI2
Br2��2I��===2Br����I2
��KIO3�ܽ�I������ΪI2��Ҳ�ܽ�Br��������Br2���䱾������ԭΪI2
IO3����5I����6H��===3I2��3H2O 2IO3����10Br����12H��===I2��5Br2��6H2O
(1)��������Ӧ�漰����������������ǿ����________��
(2)��KI��KBr�Ļ����Һ�У����������KBrO3������������Ϊ________����ԭ����Ϊ________��
(3)����1 mol KI��������Һ�м���KBrO3��Һ����Ӧ���Ԫ��ֻ������I2�У���Ԫ��ֻ������Br���У������KBrO3�����ʵ���Ϊ________��
(4)��6 mL 0.4 mol��L��1 KBrO3��Һ��10 mL 0.4 mol��L��1KI��Һ��ϡH2SO4�л�ϡ�д��������Ӧ�����ӷ���ʽ______________________________________________________��
(4)10I����6BrO3����12H��===3Br2��3I2��4IO3����6H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�������ѧ�ڻ�ѧһ�ָ�ϰ���Ӻ�ˮ�л�û�ѧ���ʡ�ר���ۺϲ��ԣ��ս̰棩 ���ͣ������
(9��)��֪�����������������·�Ӧ��ϵ��
��KBrO3�ܽ�KI������I2��KlO3���䱾������ԭΪBr2
2BrO3����10I����12H��===5I2��Br2��6H2O 6BrO3����5I����6H��===5IO3����3Br2��3H2O
��Br2�ܽ�I������ΪI2
Br2��2I��===2Br����I2
��KIO3�ܽ�I������ΪI2��Ҳ�ܽ�Br��������Br2���䱾������ԭΪI2
IO3����5I����6H��===3I2��3H2O 2IO3����10Br����12H��===I2��5Br2��6H2O
(1)��������Ӧ�漰����������������ǿ����________��
(2)��KI��KBr�Ļ����Һ�У����������KBrO3������������Ϊ________����ԭ����Ϊ________��
(3)����1 mol KI��������Һ�м���KBrO3��Һ����Ӧ���Ԫ��ֻ������I2�У���Ԫ��ֻ������Br���У������KBrO3�����ʵ���Ϊ________��
(4)��6 mL 0.4 mol��L��1 KBrO3��Һ��10 mL 0.4 mol��L��1KI��Һ��ϡH2SO4�л�ϡ�д��������Ӧ�����ӷ���ʽ______________________________________________________��
(4)10I����6BrO3����12H��===3Br2��3I2��4IO3����6H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪�����������������·�Ӧ��ϵ��
��KBrO3�ܽ�KI������I2��KIO3���䱾������ԭΪBr2��
2BrO3��+10I��+12H+ = 5I2 +Br2 + 6H2O 6BrO3��+5I�� +6H+ = 5IO3��+3Br2+3H2O
��Br2�ܽ�I������ΪI2��Br2+2I�� = 2Br��+I2
��KIO3�ܽ�I������ΪI2��Ҳ�ܽ�Br��������Br2���䱾������ԭΪI2��
IO3��+5I�� + 6H+ = 3I2 + 3H2O 2IO3��+ 10Br��+12H+ = I2 + 5Br2 + 6H2O
��1����������Ӧ�漰����������������ǿ���� ���ѧʽ����ͬ����
��2����KI��KBr�Ļ����Һ�У����������KBrO3������������Ϊ ����ԭ����Ϊ ��
��3������1mol KI��������Һ�м�KBrO3��Һ������Ӧ��ĵ�Ԫ��ֻ������I2�У���Ԫ��ֻ����Br���У������KBrO3�����ʵ���Ϊ mol��
��4����6mL 0.4mol��L��1KBrO3��Һ��10mL 0.4mol��L��1KI��Һ��ϡH2SO4�л�ϡ�д��������Ӧ�����ӷ���ʽ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
I2��KI��Һ�д�
������ƽ�⣺I2(aq)��I��(aq)![]() I3��(aq) ��H =Q ��ijI2����KI�����Һ�У�I3�������ʵ���Ũ��c(I3��)���¶�T�Ĺ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������˵����ȷ����
I3��(aq) ��H =Q ��ijI2����KI�����Һ�У�I3�������ʵ���Ũ��c(I3��)���¶�T�Ĺ�ϵ��ͼ��ʾ���������κ�һ�㶼��ʾƽ��״̬��������˵����ȷ���� 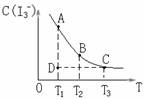
A����Ӧ I2(aq)��I��(aq)![]() I3��(aq) ��H =Q�� Q��0
I3��(aq) ��H =Q�� Q��0
B�����¶�ΪT1��T2����Ӧ��ƽ�ⳣ���ֱ�ΪK1��K2��K2��K1
C������Ӧ���е�״̬Dʱ��һ����v����v��
D��״̬A��״̬B��ȣ�״̬A��c(I2)��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com