
��NA��ʾ�����ӵ�������ֵ������˵����ȷ���ǣ� ��
A��0��1L��2mol/L�ģ�NH4��2CO3��Һ�к���CO32-��ĿΪ0��2NA
B�����������£�MnO2��Ũ���ᷴӦ����7��1gCl2��ת�Ƶĵ�����ĿΪ0��2NA
C����״���£�11��2LCCl4�к��еķ�����ĿΪ0��5NA
D�������£�1L0��1mol•L-1��CH3COOH��Һ�к��е�H+��ĿΪ0��1NA

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017���Ĵ�ʡ�¸�������ģ�����ۻ�ѧ�Ծ��������棩 ���ͣ������
�ݱ�����һ�������� Fe2O3�ɱ����黹ԭΪ���������Ľ��������䷴ӦΪ�� Fe2O3(s)+3CH4(g)  2Fe(s)+3CO(g)+6H2(g)
2Fe(s)+3CO(g)+6H2(g)
��1����Ӧ��5 L���ܱ������н��У�2 min ��ﵽƽ�⣬��� Fe2O3�ڷ�Ӧ���������� 4.8 g����ö�ʱ����H2��ƽ����Ӧ����Ϊ______________��
��2�����̶������� Fe2O3(s)�� CH4(g)���ں��º�ѹ�����У���һ�������·�Ӧ���ܱ����÷�Ӧ�ﵽƽ��״̬����______________��
A��CH4��ת���ʵ��� CO�IJ���
B����������ƽ����Է�����������
C��v ��(CO): v ��(H2)=1 : 2
D�����������������
��3��FeO ����CO���л�ԭ����֪��t��ʱ��FeO(s)+CO(g)  Fe(s)+CO2(g) K=0.5
Fe(s)+CO2(g) K=0.5
���� 1 L�ܱ������м���0.04 mol FeO(s)����ͨ�� x mol CO��t��ʱ��Ӧ�ﵽƽ�⡣��ʱFeO(s)��ת����Ϊ 50%����x=______________��
��4���� 3 L�ݻ��ɱ���ܱ������з�����Ӧ��FeO(s)+CO(g)  Fe(s)+CO2(g)��c(CO2)�淴Ӧʱ��t�仯��ͼ������I��ʾ������t0ʱ�̷ֱ�ı�һ�������� ���� I��Ϊ����II������III��������I��Ϊ���� IIʱ���ı��������______________����ͨ���ı�ѹǿʹ���� I��Ϊ����IIIʱ������ III�ﵽƽ��ʱ���������Ϊ_________L��
Fe(s)+CO2(g)��c(CO2)�淴Ӧʱ��t�仯��ͼ������I��ʾ������t0ʱ�̷ֱ�ı�һ�������� ���� I��Ϊ����II������III��������I��Ϊ���� IIʱ���ı��������______________����ͨ���ı�ѹǿʹ���� I��Ϊ����IIIʱ������ III�ﵽƽ��ʱ���������Ϊ_________L��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�����ɹŸ߶�ʵ��������л�ѧ���������棩 ���ͣ�ѡ����
���и������ʵľ����У���ѧ��������ͬ����������Ҳ��ͬ���ǣ� ��
A��SO2��SiO2 B��CO2��H2O C��NaCl��HCl D��KCl��CCl4
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶�����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
������Һ���������ʵ���Ũ�ȹ�ϵ��ȷ����
A��������0��1 mol/L��������Һ�� NH4Al��SO4��2���� NH4Cl���� NH3��H2O���� CH3COONH4��Һ�У�c��NH4+���ɴ�С��˳���Ǣڣ��٣��ܣ���
B��������0��4 mol/L CH3COOH��Һ��0��2 mol/L NaOH��Һ�������Ϻ���Һ�����ԣ�����Һ������Ũ�ȴ�С˳��Ϊ��c��CH3COOH����c��Na+����c��CH3COO������c��H+����c��OH����
C��0��1 mol/L��NH4��2Fe��SO4��2��Һ��c��NH4+��+c��NH3��H2O��+ c��Fe2+��=0��3 mol/L
D�������£���0��1 mol/LNH4HSO4��Һ�еμ�NaOH��Һ�����ԣ�c��Na+����c��SO42-����c��NH4+����c��OH���� = c��H+��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶�����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
��б���������ת��Ϊ��������������仯��ͼ��ʾ������˵����ȷ���ǣ� ��
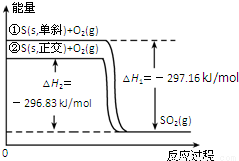
A����б��ת��Ϊ������ķ�Ӧ�����ȷ�Ӧ
B��������ȵ�б���ȶ�
C����ͬ���ʵ�����������ȵ�б�������е�������
D���ٱ�ʾ����1mol O2�еĹ��ۼ������յ��������γ�lmolSO2�еĹ��ۼ����ų���������297��16 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ�߶�����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
���б�ʾ���ʽṹ�Ļ�ѧ�����ģ����ȷ����
A����������Ľṹ��ʽ��C2H4O2
B��H2O2�ĵ���ʽ��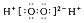
C��������Ϊ10 ����ԭ�ӣ�188O
D����-�����ױ��Ľṹ��ʽ��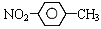
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������տ���һ�и߶��¿���ѧ�Ծ��������棩 ���ͣ������
������Ҵ������������ֳ������л���������Ҵ��ɷ������·�Ӧ��
CH3COOH+HOC2H5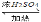 CH3COOC2H5+H2O
CH3COOC2H5+H2O
��1���÷�Ӧ������_______________��Ӧ(�ȡ�����ӳɡ�)��
��2�����������ԣ��������������_____________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�������տ���һ�и߶��¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����������������ʱ���ᷢ����ɫ�仯����
A��N2 B��NH3 C��CO2 D��NO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ɽ��ʡ�߶�����ĩ��ѧ�Ծ��������棩 ���ͣ�ѡ����
���з����У����ڷǼ��Է��ӵ���
A��SO2 B��BeCl2 C��NF3 D��COCl2
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com