
�������ʵij��ӷ����У���������(����)
��C2H6�л��е�C2H4�����Խ����������ͨ����ˮ��Ȼ���ü�ʯ�Ҹ���
��K2CO3�л��е�����NaHCO3�������ü��ȵķ�����ȥ
����ϩ�л��е�SO2�������ͨ�����Ը��������Һ��ȥ
�ܽ���ͭ�к��е���������п����ͨ����⾫���ķ�����ȥ
��H2S�л��е�ˮ������Ũ������T��
A���٢ܡ����������� B���ڢ�
C���٢� D���ܢ�
��������������C2H4��C2H6����ͨ����ˮ��C2H4���巢���ӳɷ�Ӧ������ȥ����ͨ����ʯ�Ҹ��T�ɵõ�C2H6���ٶԣ�����NaHCO3��K2CO3������ʱNaHCO3�ֽ�ת��ΪNa2CO3�����յõ�K2CO3��Na2CO3�Ļ����ڴ�����ϩ��SO2���ܱ����Ը��������Һ�������۴�����⾫����Ag��Zn�Ĵ�ͭʱ��Zn�ŵ�������Һ��Cu�ŵ�������Һ��Cu2���������Ϸ�Ӧ�õ���ͭ��Ag�γ������࣬�ܶԣ�H2S���л�ԭ�ԣ��ܱ�Ũ�����������ݴ���
���𰸡���A


 ����ѧ��Ӯ�����ϵ�д�
����ѧ��Ӯ�����ϵ�д� ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
ѧ���쳵�����ּ��������ҵ�½����������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ݱ�����ȫ��ÿ�귢��������ʴ����ɵ�ֱ�Ӿ�����ʧ����ǧ����Ԫ�����и��缫��Ӧ
ʽ�У��ܱ�ʾ���ĵ绯ѧ��ʴ���� �� ��
�� Fe��2e-=Fe2+ �� 2H++2e-=H2�� �� Fe��3e-=Fe3+
�� 2H2O+O2+4e-=4OH- �� 4OH-��4e-=2H2O+O2��
��A.�٢ڢ� B.�ڢۢ� C.�٢ڢ� D.�٢ۢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���з��ӻ�������ָ���ķ�ɢϵ���ܴ��������һ����
A��ʹ��̪���ɫ����Һ��Na����Ba2����Cl—��NO3��
B�������� C2H6��CO2��SO2��NO
C���Ȼ�����Һ�� H+��K+��NO3����I—
D�����������Һ�� H+��Na+��SO42-�������Ƿ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪X��Y��Z��W����Ԫ��ԭ�����������������ǵĽṹ��������Ϣ���±���
| Ԫ�� | �ṹ��������Ϣ |
| X | �������������ڴ��������� |
| Y | �����ڳ������ǹ��壬��̬ԭ�ӵ�M������1��δ�ɶԵ�p���� |
| Z | Ԫ�����������ӵ�3d�ܼ�Ϊ����� |
| W | Ԫ�ػ�̬ԭ�ӵ�M��ȫ������N��ֻ��һ������ |
��1��Ԫ��Y��ԭ�Ӻ���� �ֲ�ͬ�˶�״̬�ĵ��ӡ�
��2��ZԪ�ػ�̬ԭ�ӵļ۵����Ų�ʽ ������ ��
��3����������Ԫ�صĵ����п���NaOH��Һ��Ӧ���� ������������Ԫ�ط��ţ�
��4����WSO4��Һ���μӰ�ˮ���۲쵽 ��
��ص����ӷ���ʽ ��
��5������ͭ˿ʱ�������ɫ���棬ԭ���� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������Һ�У���������һ���ܹ������������(����)
A����ʹ�㷺pH��ֽ����ɫ����Һ��K����Ba2����Cl����Br��
B��������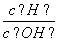 ��1012����Һ��Fe2����Mg2����NO
��1012����Һ��Fe2����Mg2����NO ��Cl��
��Cl��
C�����д���Al3������Һ��Na����Cl����AlO ��OH��
��OH��
D����ʹ���۵⻯����ֽ����ɫ����Һ��K����SO ��S2����SO
��S2����SO
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
̼��Ƴ����������Ħ������ijͬѧ�����һ���Ʊ�̼��Ƶķ�����������ͼ���£�(����ʯ��ʯ��������SiO2)

�ش��������⣺
(1)�������110��ʯ��ʯ�õ�����66�֡���״�������ɶ�����̼�����Ϊ______________L��ʯ��ʯ��̼��Ƶ���������Ϊ______________%��
(2)����ڢٲ���Ӧ��ȫ���У���ڢڲ���Ӧ���˺�õ��IJ����������ijɷ�Ϊ________________________��
(3)�ڢ۲���Ӧһ�㲻����ͨ��CO2����Ҫԭ����______________________��
��Ӧ�����ӷ���ʽΪ_____________________________________��
(4)CaCO3��һ���������ʣ�25��ʱ��Ksp��2.8��10��9���ֽ��������CaCl2��Һ��Na2CO3��Һ��ϣ���Na2CO3��Һ��Ũ��Ϊ2.0��10��4 mol/L�������ɳ�������CaCl2��Һ�����ʵ���Ũ����С��______________��
(5)ijѧ����ʯ��ʯΪԭ�ϣ��������һ���Ʊ�̼��Ƶ�ʵ�鷽����������ͼ���£�
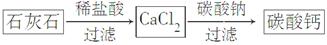
��ǰһ������Ƚϣ��÷������ŵ���_________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ڳ����л����˵������ȷ����(����)
A�����ۡ������ʡ���֬��������Ȼ�߷��ӻ�����
B������͵����ʶ���������Ҫ��Ӫ������
C����ϩ�ͼ���������Ը��������Һ����
D���������֬����������������Һ��Ӧ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
��CH3OH(g)��H2O(g)===CO2(g)��3H2(g)
��H1����49.0 kJ/mol��
��CH3OH(g)�� O2(g)===CO2(g)��2H2(g)
O2(g)===CO2(g)��2H2(g)
��H2����192.9 kJ/mol��
����������Ӧ������˵����ȷ����(����)
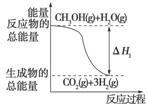
A����Ӧ���е������仯����ͼ��ʾ
B��CH3OHת���H2�Ĺ���һ��Ҫ��������
C��1 mol CH3OH���ȼ�շų�������Ϊ192.9 kJ
D������֪2H2(g)��O2(g)===2H2O(g)
��H����483.8 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����˵����ȷ���� ( )
A��Ca2+�ĺ�������Ų�ʽΪ1s22s22p63s23p6 4s2
B����ԭ�ӵļ۵����Ų�ͼΪ��
C��ijԭ�ӵĺ�������Ų�ʽΪ1s22s22p63s23p63d54s1 ������ɵ�������Ϊ2
D�����ܼ���ԭ�ӹ������s��p��d��f��˳������Ϊ1��3��5��7
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com