
����Cr�������ؽ���Ԫ�أ������ķ�ˮ�ͷ����ŷű��뾭�������ﵽ�йصİ�ȫ����
��1����ԭ�Ӻ���������Ϊ24����λ�����ڱ��е�______���ڡ�
��2����Ԫ����Cr2O72-����ʽ���������Է�ˮ�У�����FeSO4���仹ԭΪCr3�������ó��������з��롣��֪��
��FeSO4��ԭCr2O72-�����ӷ���ʽΪ______________________________________________��
��Cr2��SO4��3��Һ�м������NaOHŨ��Һ����Ӧ�����ӷ���ʽΪ_____________________��
�۳����������ˮ�е�Cr3����pHӦ���Ƶķ�Χ��________��
�����й��ڸ����仯�����˵������ȷ����________��
A��K2Cr2O7��һ�ֳ��õ�ǿ������
B������K2Cr2O7��Һ�����ڼ���˾���Ƿ�ƺ�ݳ�
C��������Ӳ�ȴ���ʴ���dz��õĶƲ����

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����йػ�ѧ����ı�ʾ��ȷ����
A��������Ϊ20����ԭ�ӷ���Ϊ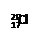 |
| B����ϩ�Ľṹ��ʽΪCH2CH2 |
| C��C60��ʯī��Ϊͬλ�� |
| D��NaHCO3�ĵ��뷽��ʽΪNaHCO3��Na��+ HCO3- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�����ܴ����������������
| A��K����[Al(OH)4]����NO3����Ba2�� | B��Na����NH4����Cl����OH�� |
| C��K����Mg2����SO42����CO32�� | D��H����Na����HCO3����SO42�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ӷ���ʽ��д��ȷ���ǣ� ��
A��ͭ����Ũ����:Cu+4HNO3��Ũ�� Cu2++2NO2��+2H2O Cu2++2NO2��+2H2O |
B����������Һ������������Һ���:Fe3++S +Ba2++3OH- +Ba2++3OH- Fe��OH��3��+BaSO4�� Fe��OH��3��+BaSO4�� |
C�����������Һ��ͨ�������CO2:ClO-+H2O+CO2 HC HC +HClO +HClO |
D��̼����þ��������ʯ��ˮ��Ӧ:Mg2++2HC +2OH-+Ca2+ +2OH-+Ca2+ CaCO3��+MgCO3��+2H2O CaCO3��+MgCO3��+2H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�ס��ҡ�����������������ˮ�����ʣ��ֱ���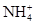 ��Ba2+��Mg2+��H+��OH-��Cl-��
��Ba2+��Mg2+��H+��OH-��Cl-��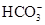 ��
��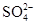 �еIJ�ͬ�����Ӻ������Ӹ�һ����ɣ���֪��
�еIJ�ͬ�����Ӻ������Ӹ�һ����ɣ���֪��
�ٽ�����Һ�ֱ��������������ʵ���Һ��ϣ����а�ɫ�������ɣ���0.1 mol/L����Һ��c��H+��>0.1 mol/L������������е���AgNO3��Һ�в�����ϡ����İ�ɫ�������ɣ����н��۲���ȷ���ǣ� ��
| A������Һ����Ba2+ | B������Һ����SO42+ |
| C������Һ����Cl- | D������Һ����Mg2+ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
��������Һ�У���������һ���ܹ�����������ǣ�������
| A����ʹ�㷺pH��ֽ����ɫ����Һ��K����Ba2����Cl����Br�� |
B�������� ��1012����Һ��Fe2����Mg2����NO3-��Cl�� ��1012����Һ��Fe2����Mg2����NO3-��Cl�� |
| C�����д���Al3������Һ��Na����Cl����AlO2-��OH�� |
| D����ʹ���۵⻯����ֽ����ɫ����Һ��K����SO42-��S2����SO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
�������ӷ���ʽ��д����ȷ���ǣ���������
| A��NaOH��Һ��SO2��Ӧ����n��NaOH����n��SO2����4��3ʱ��4OH����3SO2=SO32-��2HSO3-��H2O |
| B��CuCl2��Һ��Na2S��Һ2��1��Ӧ��Cu2����S2��=CuS�� |
| C��Cl2��FeBr2��Һ��Ӧ����n��Cl2����n��FeBr2����1��1ʱ��2Fe2����4Br����3Cl2=2Fe3����2Br2��6Cl�� |
| D��1 mol��L��1��NaAlO2��Һ��2.5 mol��L��1����������������Ȼ�ϣ�2AlO2-��5H��=Al��OH��3����Al3����H2O |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����ȷ��ʾ���з�Ӧ�����ӷ���ʽ��(����)
| A���ù�����ˮ���չ�ҵβ���е�SO2��2NH3��H2O��SO2=2NH4+��SO32����H2O |
| B���Ȼ�����Ũ�����ϼ��ȣ�H2SO4��2Cl����,SO2����Cl2����H2O |
| C����������������ϡ���3Fe2����4H����NO3��=3Fe3����NO����3H2O |
| D��������Һ�е���Ba(OH)2��ҺʹSO42��ǡ����ȫ������2Ba2����3OH����Al3����2SO42��=2BaSO4����Al(OH)3�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ���ѡ��
����A��D���飬ÿ����������Ӧ������������Ӧ����ͬһ�����ӷ���ʽ��ʾ���ǣ� ��
| | ���� | ���� |
| A | ����SO2ͨ��Ba��OH��2��Һ�� | ����SO2ͨ������Ba��OH��2��Һ�� |
| B | ����Ũ��ˮ����Al2��SO4��3��Һ�� | ����Al2��SO4��3��Һ����Ũ��ˮ�� |
| C | 0.1 mol Cl2ͨ�뺬0.2 mol FeBr2����Һ�� | 0.3 mol Cl2ͨ�뺬0.2 mol FeBr2����Һ�� |
| D | ����BaCl2��Һ������Na2SO4��Һ���� | ����Ba��OH��2��Һ�����MgSO4��Һ���� |
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com