
| n |
| V |
 ��KIΪ��ԭ����+5�۵�IԪ�ر���ԭ����ԭ���������������Ϊ�⣬��ԭ���غ㼰��Ӧ��֪�����ʵ���֮��Ϊ1��5���ʴ�Ϊ��IO3-+5I-+6H+=3I2+3H2O��5��1��
��KIΪ��ԭ����+5�۵�IԪ�ر���ԭ����ԭ���������������Ϊ�⣬��ԭ���غ㼰��Ӧ��֪�����ʵ���֮��Ϊ1��5���ʴ�Ϊ��IO3-+5I-+6H+=3I2+3H2O��5��1��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�


�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| 1 |
| 2 |
| ʵ���� | �¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
| H2O | CO | H2 | CO2 | |||
| 1 | 650 | 2 | 4 | 1.6 | 1.6 | 5 |
| 2 | 900 | 1 | 2 | 0.4 | 0.4 | 3 |
| 3 | 900 | a | b | c | d | t |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��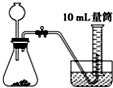 |
B��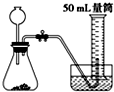 |
C��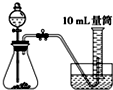 |
D��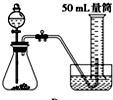 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| A����ˮ��ʯ�������ܵ�ԭ�����ܽ���CO2����ˮ��CaCO3���������˿�����Ca��HCO3��2��Ե�� |
| B��25��ʱ��������XY��AB3��Ksp�ֱ�Ϊ1.0��10-10��2.7��10-15������¶��£����ߵı�����Һ��c��X+��һ������c��A3+�� |
| C��25��ʱ��pH=ll��KaA��Һ��pH=11��KOH��Һ��ˮ�����c��OH-��ǰ���Ǻ��ߵ�108�� |
| D�����������Լ��ܰ�NaCl��AlCl3��Ba��OH��2������Һ������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��ʾ��ͬ��һ��ԭ�ӣ�������֮������ߴ�����ѧ�����絥����˫���ȣ���
��ʾ��ͬ��һ��ԭ�ӣ�������֮������ߴ�����ѧ�����絥����˫���ȣ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| ʵ����� | ʱ�� Ũ�� �¶� | 0 | 10 | 20 | 30 | 40 | 50 | 60 |
| 1 | 800 | 1.0 | 0.80 | 0.67 | 0.57 | 0.50 | 0.50 | 0.50 |
| 2 | 800 | 1.0 | 0.60 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| 3 | 800 | 1.0 | 0.40 | 0.20 | 0.20 | 0.20 | 0.20 | 0.20 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
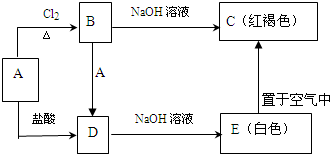
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com