
| A | B | D | E | F |
| ����C��H��O ����Ԫ����ɵ�Һ�壻 ����ˮ����������ܡ� | �������������һ�����ҵ�ʯ�ͻ���ˮƽ�� | ����ģ��Ϊ�� | �ٿ����ڳ�ˮ�� ���ڵ���16.6��ʱ�����̳ɱ�һ���ľ��塣 | ��5��ԭ����ɵ�10�����ӵĹ��۷��ӡ� |

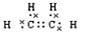 ���ӳɣ�18.8 ����1�֣���3�֣�
���ӳɣ�18.8 ����1�֣���3�֣�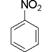 ����1�֣���2�֣�
����1�֣���2�֣�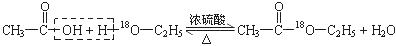 ��2�֣��� 4��1�֣�
��2�֣��� 4��1�֣�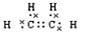 ����5��ԭ����ɵ�10�����ӵĹ��۷���Ӧ���Ǽ��飬��F�Ǽ��顣�������ˮ����Ӧ����ϩ����ˮ�����ӳɷ�Ӧ����ˮ���ӵ�����������ϩ������������ϩ��2.8g�����ʵ�����0.1mol������0.1molCH2BrCH2Br��������18.8g��
����5��ԭ����ɵ�10�����ӵĹ��۷���Ӧ���Ǽ��飬��F�Ǽ��顣�������ˮ����Ӧ����ϩ����ˮ�����ӳɷ�Ӧ����ˮ���ӵ�����������ϩ������������ϩ��2.8g�����ʵ�����0.1mol������0.1molCH2BrCH2Br��������18.8g��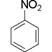 ��
��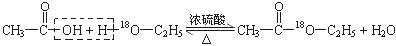 ������װ��ͼ��֪������Ҫ�þƾ���ƣ��ռ�װ����û���ñ���̼������Һ�����Һû�л�Ͼ��ȣ��Թ��еĵ��ܿ�¶����̫�������Թ�����4������
������װ��ͼ��֪������Ҫ�þƾ���ƣ��ռ�װ����û���ñ���̼������Һ�����Һû�л�Ͼ��ȣ��Թ��еĵ��ܿ�¶����̫�������Թ�����4������

 ÿ��10���ӿ�����������������ϵ�д�
ÿ��10���ӿ�����������������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ʴ���
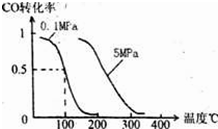
 CO2+H2���ﵽƽ��ʱ��
CO2+H2���ﵽƽ��ʱ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
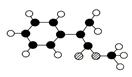
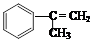 �������ϳ�M����ϳ�·�����£�
�������ϳ�M����ϳ�·�����£�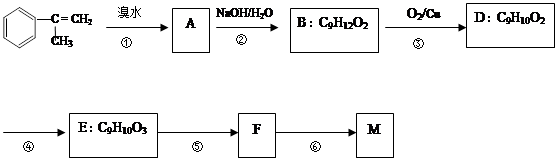
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���ܷ����ӳɡ�ȡ����Ӧ |
| B���ܷ�����ԭ��������Ӧ |
| C�������ڹ���19����ԭ�� |
| D�������ڹ�ƽ���̼ԭ�Ӷ���6�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
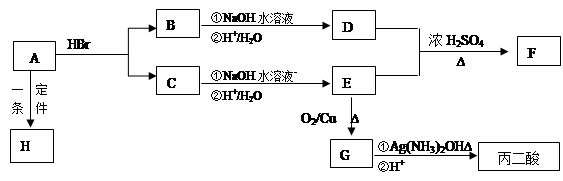
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������


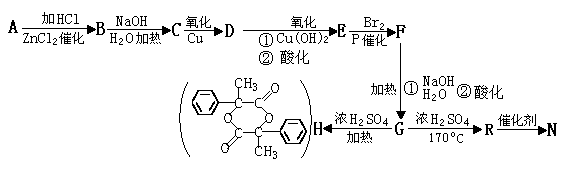
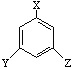 ������������Ӧ����FeCl3��Һ���ܳ���ɫ��ͬ���칹��Ľṹ��ʽ ��
������������Ӧ����FeCl3��Һ���ܳ���ɫ��ͬ���칹��Ľṹ��ʽ ���鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com