
��ϩ����Ҫ�Ļ���ԭ�ϣ��Ա�ϩΪԭ�Ϻϳ�ij���л���������ī����DAP-A��֬����������
�ش����⣺
��1��д���������ʿ��ܵĽṹ��ʽ��B_________________��F_________________��
��2��д����Ӧ���ͣ���Ӧ��______________����Ӧ��____________________��
��3��1 mol DAP-A��֬��һ����������H2�����ӳɷ�Ӧ���������H2______mol��
��4��д����Ӧ�ٵĻ�ѧ����ʽ___________________________________��
��5��д��G��һ�����������Ҷ���������Ӧ����һ�ָ߷��ӻ�����Ļ�ѧ����ʽ ��
��6��E��ͬ���칹���У������������״�л�������
��___________________________________��
 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| H2O/H+ |

 ������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ
������NaOH��Һ��Ӧ�Ļ�ѧ����ʽΪ +2NaOH
+2NaOH +H2O+NaBr
+H2O+NaBr +2NaOH
+2NaOH +H2O+NaBr
+H2O+NaBr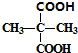 +2C2H5OH
+2C2H5OH| ŨH2SO4 |
| �� |
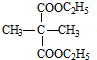 +2H2O
+2H2O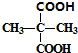 +2C2H5OH
+2C2H5OH| ŨH2SO4 |
| �� |
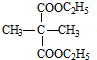 +2H2O
+2H2O�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ��1��1mol O2������������Ӧ����ˮ��������483.6kJ����1gˮ����ת����Һ̬ˮ����2.444kJ����д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ��
��1��1mol O2������������Ӧ����ˮ��������483.6kJ����1gˮ����ת����Һ̬ˮ����2.444kJ����д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ϩ����Ҫ�Ļ���ԭ�ϣ��Ա�ϩΪԭ�Ϻϳ��л���������ī����DAP-A��֬���������£�

�ش����⣺
��1��д���������ʿ��ܵĽṹ��ʽ��B___________________��F________________________��
��2��д����Ӧ���ͣ���Ӧ��___________________����Ӧ��____________________________��
��3��1 mol DAP-A��֬��һ����������H2�����ӳɷ�Ӧ���������H2_______________mol��
��4��д����Ӧ�ٵĻ�ѧ����ʽ______________________________________________��
��5��д��G��һ�����������Ҷ���������Ӧ����һ�ָ߷��ӻ�����Ļ�ѧ����ʽΪ_______��
��6��E��ͬ���칹���У������������״�л�����

![]() �� ��___________________________________��
�� ��___________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ĵ�ʡ�ɶ�����������9��ͳһ��⻯ѧ�Ծ����������� ���ͣ������
( 16 ��)��ϩ����Ҫ�Ļ���ԭ�ϣ��Ա�ϩΪԭ�Ϻϳ��л���������ī����DAP-A��֬���������£�
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ĵ�ʡ�ɶ�����������9��ͳһ��⻯ѧ�Ծ��������棩 ���ͣ������
( 16 ��)��ϩ����Ҫ�Ļ���ԭ�ϣ��Ա�ϩΪԭ�Ϻϳ��л���������ī����DAP-A��֬���������£�

|
��1��д���������ʿ��ܵĽṹ��ʽ��
B________________ ___��F____________ ____________��
��2��д����Ӧ���ͣ���Ӧ��_______________����Ӧ��____________________________��
��3��1 mol DAP-A��֬��һ����������H2�����ӳɷ�Ӧ���������H2_______________mol��
��4��д����Ӧ�ٵĻ�ѧ����ʽ��
_______________ _________��
��5��д��G��һ�����������Ҷ���������Ӧ����һ�ָ߷��ӻ�����Ļ�ѧ����ʽΪ
__ ____��
��6��E��ͬ���칹���У�����������û��˳���칹����״�л����У�д�ṹ��ʽ����
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com