
 _______��
_______��| A����ʹ���Ը��������Һ��ɫ | B����ʹ���CCl4��Һ��ɫ |
| C��һ�������£��ܹ�������ȥ��Ӧ | D��һ�������£��ܹ�����ȡ����Ӧ |
 ��������ϵ�д�
��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 2CH3COOH��
2CH3COOH��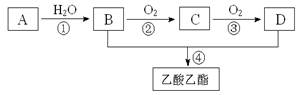
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��ʹ������Ȼ�̼��Һ��ɫ���ش����⣺
��ʹ������Ȼ�̼��Һ��ɫ���ش����⣺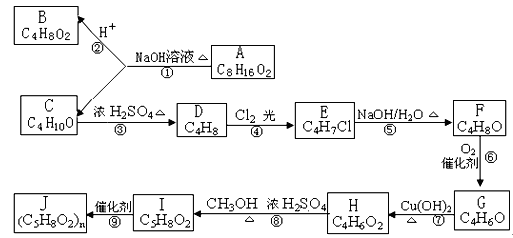
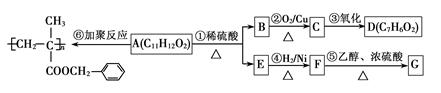
 ����д������ȥ��Ӧ�г�D�����һ������Ľṹ��ʽ__________________________��
����д������ȥ��Ӧ�г�D�����һ������Ľṹ��ʽ__________________________�� ���������־ۺϲ���Ľṹ��ʽ��____________________________________________________________��
���������־ۺϲ���Ľṹ��ʽ��____________________________________________________________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
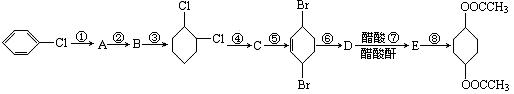
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
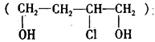 �Ǻϳɸ߷��ӻ�����HPMA���м��壬HPMA�����ڹ�¯�蹸����
�Ǻϳɸ߷��ӻ�����HPMA���м��壬HPMA�����ڹ�¯�蹸����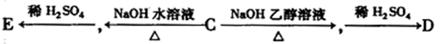
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
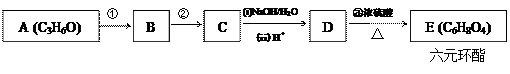
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
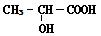 ����һ�����������ɾ�����ķ�Ӧ����ʽ
����һ�����������ɾ�����ķ�Ӧ����ʽ �鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 �ʵĽṹ��ʽ�ֱ�Ϊ��
�ʵĽṹ��ʽ�ֱ�Ϊ���鿴�𰸺ͽ���>>
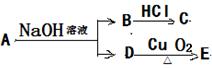
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 L�� NaOH��Һ��ȫ��Ӧ��A�к���һ��һCOOH��һ��һCH3,�����������ᷢ��������Ӧ;C��ϡ�����ϡ����������Һ�о��ܷ�Ӧ�����������գ�
L�� NaOH��Һ��ȫ��Ӧ��A�к���һ��һCOOH��һ��һCH3,�����������ᷢ��������Ӧ;C��ϡ�����ϡ����������Һ�о��ܷ�Ӧ�����������գ�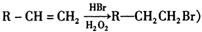 _____________________��______________
_____________________��______________�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com