
(8��)�л�������A��̼�ĺ���Ϊ90.57%�������Ϊ�⣬���л���Կ���������ܶ�Ϊ3.66
(1)���л������Է�������
(2)���л���ķ���ʽΪ
(3)���л��ﺬ������ͬ���������� �֡�
(4)��������ʵ�������ۣ�ȷ�����л���Ľṹ��
������������ˮ��Ӧ��
������ʹKMnO4������Һ��ɫ��
�����ܷ���������Ӧ��������1������ʱ��ֻ�ܵõ�1�����������
���л���Ľṹ��ʽΪ

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��ͼ��A��B��C��D��E��F��G��H��Ϊ�л������

�ش��������⣺
��1���л������� A����Է�������С��60��A�ܷ���������Ӧ��1molA�ڴ�������������3 mol H2��Ӧ����B����A�Ľṹ��ʽ��___________________����A����B�ķ�Ӧ������ ��
��2��B��Ũ�����м��ȿ�����C��C�ڴ��������¿ɾۺ����ɸ߷��ӻ�����D����C����D�Ļ�ѧ����ʽ��__________________________��
��3���ٷ��㻯����E�ķ���ʽ��C8H8Cl2��E�ı����ϵ�һ��ȡ����ֻ��һ�֣���E�����п��ܵĽṹ��ʽ��_______________________________________________
��E��NaOH��Һ�п�ת��ΪF��F�ø������������Һ��������G��C8H6O4����1 mol G�������� NaHCO3��Һ��Ӧ�ɷų� 44.8 L CO2����״�������ɴ�ȷ��E�Ľṹ��ʽ��__________________________________________________
��4��G��������B��Ũ������¼��ȷ�Ӧ������H������G��B����H�Ļ�ѧ����ʽ��___________________________________���÷�Ӧ�ķ�Ӧ������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2008����ͨ�ߵ�ѧУ����ͳһ�����������⻯ѧ���֣��Ĵ����� ���ͣ��ƶ���
��16�֣���ͼ��A��B��C��D��E��F��G��H��Ϊ�л������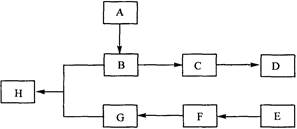
�ش��������⣺
��1���л������� A����Է�������С��60��A�ܷ���������Ӧ��1molA�ڴ�������������3 mol H2��Ӧ����B����A�Ľṹ��ʽ��___________________����A����B�ķ�Ӧ������ ��
��2��B��Ũ�����м��ȿ�����C��C�ڴ��������¿ɾۺ����ɸ߷��ӻ�����D����C����D�Ļ�ѧ����ʽ��__________________________��
��3���ٷ��㻯����E�ķ���ʽ��C8H8Cl2��E�ı����ϵ�һ��ȡ����ֻ��һ�֣���E�����п��ܵĽṹ��ʽ��_______________________________________________
��E��NaOH��Һ�п�ת��ΪF��F�ø������������Һ��������G��C8H6O4����1 mol G�������� NaHCO3��Һ��Ӧ�ɷų� 44.8 L CO2����״�������ɴ�ȷ��E�Ľṹ��ʽ��__________________________________________________
��4��G��������B��Ũ������¼��ȷ�Ӧ������H������G��B����H�Ļ�ѧ����ʽ��___________________________________���÷�Ӧ�ķ�Ӧ������________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�Ʒ���ѧ09-10ѧ��߶���ѧ��3���¿� ���ͣ������
(8��)�л�������A��̼�ĺ���Ϊ90.57%�������Ϊ�⣬���л���Կ���������ܶ�Ϊ3.66
(1)���л������Է�������
(2)���л���ķ���ʽΪ
(3)���л��ﺬ������ͬ���������� �֡�
(4)��������ʵ�������ۣ�ȷ�����л���Ľṹ��
������������ˮ��Ӧ��
������ʹKMnO4������Һ��ɫ��
�����ܷ���������Ӧ��������1������ʱ��ֻ�ܵõ�1�����������
���л���Ľṹ��ʽΪ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(8��)�л�������AΪһԪ����л��ᣬ����C��H��O��BrԪ�أ���A��صķ�Ӧ��ͼ���£�

(1)д�����з�Ӧ���л���Ӧ���ͣ�
A��C�Т��� ��Ӧ�� C��E�� ��Ӧ��
(2)д��F�Ľṹ��ʽ��
(3)д����C������ͬ�����ŵ�ͬ���칹��Ľṹ��ʽ ��
(4)д�����з�Ӧ�Ļ�ѧ����ʽ
A��B�� ��
�鿴�𰸺ͽ���>>
����ѧУ��ѡ - ��ϰ���б� - �����б�
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com